Rodent facial nerve recovery after selected lesions and repair techniques
- PMID: 20048604
- PMCID: PMC4394362
- DOI: 10.1097/PRS.0b013e3181c2a5ea
Rodent facial nerve recovery after selected lesions and repair techniques
Abstract
Background: Measuring rodent facial movements is a reliable method for studying recovery from facial nerve manipulation and for examining the behavioral correlates of aberrant regeneration. The authors quantitatively compared recovery of vibrissal and ocular function following three types of clinically relevant nerve injury.
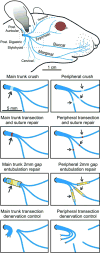
Methods: One hundred seventy-eight adult rats underwent facial nerve manipulation and testing. In the experimental groups, the left facial nerve was either crushed, transected, and repaired epineurially, or transected and the stumps suture-secured into a tube with a 2-mm gap between them. Facial recovery was measured for the ensuing 1 to 4 months. Data were analyzed for whisking recovery. Previously developed markers of co-contraction of the upper and midfacial zones (possible synkinesis markers) were also examined.
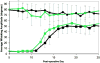
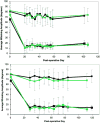
Results: Animals in the crush groups recovered nearly normal whisking parameters within 25 days. The distal branch crush group showed improved recovery over the main trunk crush group for several days during early recovery. By week 9, the transection/repair groups showed evidence of recovery that trended further upward throughout the study period. The entubulation groups followed a similar recovery pattern, although they did not maintain significant recovery levels by the study conclusion. Markers of potential synkinesis increased in selected groups following facial nerve injury.
Conclusions: Rodent vibrissal function recovers in a predictable fashion following manipulation. Generalized co-contraction of the upper and midfacial zones emerges following facial nerve manipulation, possibly related to aberrant regeneration, polyterminal axons, or hypersensitivity of the rodent to sensory stimuli following nerve manipulation.
Conflict of interest statement
The authors have no financial disclosures to make.
Figures



References
-
- Lee J, Fung K, Lownie SP, Parnes LS. Assessing impairment and disability of facial paralysis in patients with vestibular schwannoma. Arch Otolaryngol Head Neck Surg. 2007 Jan;133(1):56–60. - PubMed
-
- Ryzenman JM, Pensak ML, Tew JM., Jr Facial paralysis and surgical rehabilitation: a quality of life analysis in a cohort of 1,595 patients after acoustic neuroma surgery. Otol Neurotol. 2005 May;26(3):516–21. discussion 521. - PubMed
-
- Coulson SE, O’dwyer NJ, Adams RD, Croxson GR. Expression of emotion and quality of life after facial nerve paralysis. Otol Neurotol. 2004 Nov;25(6):1014–9. - PubMed
-
- Kahn JB, Gliklich RE, Boyev KP, Stewart MG, Metson RB, McKenna MJ. Validation of a patient-graded instrument for facial nerve paralysis: the FaCE scale. Laryngoscope. 2001 Mar;111(3):387–98. - PubMed
-
- Lohne V, Bjørnsborg E, Westerby R, Heiberg E. I want to smile. How do individuals with facial paralysis resulting from surgical removal of an acoustic neuroma cope with daily living? Vard Nord Utveckl Forsk. 1986 Spring;6(1):311–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

