4'-Chloro-3,5-dihydroxystilbene, a resveratrol derivative, induces lung cancer cell death
- PMID: 20048747
- PMCID: PMC4002696
- DOI: 10.1038/aps.2009.182
4'-Chloro-3,5-dihydroxystilbene, a resveratrol derivative, induces lung cancer cell death
Abstract
Aim: To examine the antitumor effect of 4'-chloro-3,5-dihydroxystilbene, a resveratrol derivative, on lung adenocarcinoma A549 cells.
Methods: The cytotoxic IC(50) was determined by direct cell counting. Flow cytometry, monodansylcadaverine (MDC) staining, transfection, Western blot and a proteasome activity assay were used to study the cellular mechanism of 4'-chloro-3,5-dihydroxystilbene. A xenograft nude mouse model was used to analyze the antitumor effect in vivo.
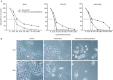
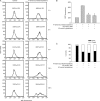
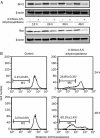
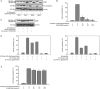
Results: 4'-Chloro-3,5-dihydroxystilbene induced a rapid and persistent increase in the intracellular reactive oxygen species in the cells, but the cell death could not be inhibited by two antioxidant agents. The derivative caused sub-G(1) formation, a decrease in the mitochondria membrane potential and poly (ADP-ribose) polymerase degradation, and the caspase inhibitor Z-VAD-FMK could partially prevent cell death. It also induced a significant increase in intracellular acidic vacuoles, LC3-II formation and intracellular GFP-LC3 aggregation. An autophagic inhibitor partially reversed cell death. Additionally, 4'-chloro-3,5-dihydroxystilbene induced the accumulation of ubiquitinated conjugates and inhibited proteasome activity in cells. In an in vivo study, 4'-chloro-3,5-dihydroxystilbene retarded tumor growth in nude mice.
Conclusion: These data suggest that the resveratrol derivative 4'-chloro-3,5-dihydroxystilbene could be developed as an anti-tumor compound.
Figures




Similar articles
-
Reactive oxygen species-mediated mitochondrial pathway is involved in Baohuoside I-induced apoptosis in human non-small cell lung cancer.Chem Biol Interact. 2012 Jul 30;199(1):9-17. doi: 10.1016/j.cbi.2012.05.005. Epub 2012 Jun 9. Chem Biol Interact. 2012. PMID: 22687635
-
Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line.Eur J Pharmacol. 2010 Sep 25;643(2-3):170-9. doi: 10.1016/j.ejphar.2010.06.025. Epub 2010 Jun 30. Eur J Pharmacol. 2010. PMID: 20599920
-
Corosolic acid induces apoptotic cell death in human lung adenocarcinoma A549 cells in vitro.Food Chem Toxicol. 2013 Jun;56:8-17. doi: 10.1016/j.fct.2013.02.002. Epub 2013 Feb 20. Food Chem Toxicol. 2013. PMID: 23454206
-
A novel polysaccharide, isolated from Angelica sinensis (Oliv.) Diels induces the apoptosis of cervical cancer HeLa cells through an intrinsic apoptotic pathway.Phytomedicine. 2010 Jul;17(8-9):598-605. doi: 10.1016/j.phymed.2009.12.014. Epub 2010 Jan 25. Phytomedicine. 2010. PMID: 20092988
-
A novel synthetic analog of Militarin, MA-1 induces mitochondrial dependent apoptosis by ROS generation in human lung cancer cells.Toxicol Appl Pharmacol. 2013 Dec 15;273(3):659-71. doi: 10.1016/j.taap.2013.10.015. Epub 2013 Oct 22. Toxicol Appl Pharmacol. 2013. PMID: 24161344
Cited by
-
Cigarette smoke-induced autophagy is regulated by SIRT1-PARP-1-dependent mechanism: implication in pathogenesis of COPD.Arch Biochem Biophys. 2010 Aug 15;500(2):203-9. doi: 10.1016/j.abb.2010.05.013. Epub 2010 May 20. Arch Biochem Biophys. 2010. PMID: 20493163 Free PMC article.
-
Effects of Resveratrol against Lung Cancer: In Vitro and In Vivo Studies.Nutrients. 2017 Nov 10;9(11):1231. doi: 10.3390/nu9111231. Nutrients. 2017. PMID: 29125563 Free PMC article. Review.
-
Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells.PLoS One. 2010 Dec 14;5(12):e15288. doi: 10.1371/journal.pone.0015288. PLoS One. 2010. PMID: 21179458 Free PMC article.
References
-
- Burns J, Yokota T, Ashihara H, Lean ME, Crozier A. Plant foods and herbal sources of resveratrol. J Agric Food Chem 2002. 50:3337–40. - PubMed
-
- Hain R, Reif HJ, Krause E, Langebartels R, Kindl H, Vornam B, et al. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature. 1993;361:153–6. - PubMed
-
- Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clin Chim Acta. 1995;235:207–19. - PubMed
-
- Djoko B, Chiou RY, Shee JJ, Liu YW. Characterization of immunological activities of peanut stilbenoids, arachidin-1, piceatannol, and resveratrol on lipopolysaccharide-induced inflammation of RAW 264.7 macrophages. J Agric Food Chem. 2007;55:2376–83. - PubMed
-
- Belguendouz L, Fremont L, Linard A. Resveratrol inhibits metal ion-dependent and independent peroxidation of porcine low-density lipoproteins. Biochem Pharmacol. 1997;53:1347–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

