Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders
- PMID: 20048751
- PMCID: PMC2914807
- DOI: 10.1038/mp.2009.144
Differential effects of prenatal and postnatal expressions of mutant human DISC1 on neurobehavioral phenotypes in transgenic mice: evidence for neurodevelopmental origin of major psychiatric disorders
Abstract
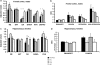
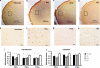
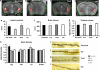
Strong genetic evidence implicates mutations and polymorphisms in the gene Disrupted-In-Schizophrenia-1 (DISC1) as risk factors for both schizophrenia and mood disorders. Recent studies have shown that DISC1 has important functions in both brain development and adult brain function. We have described earlier a transgenic mouse model of inducible expression of mutant human DISC1 (hDISC1) that acts in a dominant-negative manner to induce the marked neurobehavioral abnormalities. To gain insight into the roles of DISC1 at various stages of neurodevelopment, we examined the effects of mutant hDISC1 expressed during (1) only prenatal period, (2) only postnatal period, or (3) both periods. All periods of expression similarly led to decreased levels of cortical dopamine (DA) and fewer parvalbumin-positive neurons in the cortex. Combined prenatal and postnatal expression produced increased aggression and enhanced response to psychostimulants in male mice along with increased linear density of dendritic spines on neurons of the dentate gyrus of the hippocampus, and lower levels of endogenous DISC1 and LIS1. Prenatal expression only resulted in smaller brain volume, whereas selective postnatal expression gave rise to decreased social behavior in male mice and depression-like responses in female mice as well as enlarged lateral ventricles and decreased DA content in the hippocampus of female mice, and decreased level of endogenous DISC1. Our data show that mutant hDISC1 exerts differential effects on neurobehavioral phenotypes, depending on the stage of development at which the protein is expressed. The multiple and diverse abnormalities detected in mutant DISC1 mice are reminiscent of findings in major mental diseases.
Figures



References
-
- European Network of Schizophrenia Networks for the Study of Gene-Environment Interactions Leboyer M, Meyer-Lindenberg A, Stefanis N, Rutten BP, Arango C, Jones P, et al. Schizophrenia aetiology: do gene-environment interactions hold the key. Schizophr Res. 2008;102:21–26. - PubMed
-
- Sanches M, Keshavan MS, Brambilla P, Soares JC. Neurodevelopmental basis of bipolar disorder: a critical appraisal. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1617–1627. - PubMed
-
- Eastwood SL. The synaptic pathology of schizophrenia: is aberrant neurodevelopment and plasticity to blame. Int Rev Neurobiol. 2004;59:47–72. - PubMed
-
- Keshavan MSGAR, Diwadkar VA.Neurodevelopmental theoriesIn: Lieberman JA STS, Perkins DO (eds). The American Psychiatric Publishing Textbook of Schizophrenia The American Psychiatric Publishing: Arlington, VA; 200666–84.
-
- Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

