Revisiting host preference in the Mycobacterium tuberculosis complex: experimental infection shows M. tuberculosis H37Rv to be avirulent in cattle
- PMID: 20049086
- PMCID: PMC2795854
- DOI: 10.1371/journal.pone.0008527
Revisiting host preference in the Mycobacterium tuberculosis complex: experimental infection shows M. tuberculosis H37Rv to be avirulent in cattle
Abstract
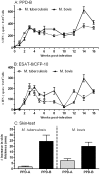
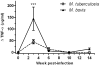
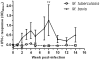
Experiments in the late 19th century sought to define the host specificity of the causative agents of tuberculosis in mammals. Mycobacterium tuberculosis, the human tubercle bacillus, was independently shown by Smith, Koch, and von Behring to be avirulent in cattle. This finding was erroneously used by Koch to argue the converse, namely that Mycobacterium bovis, the agent of bovine tuberculosis, was avirulent for man, a view that was subsequently discredited. However, reports in the literature of M. tuberculosis isolation from cattle with tuberculoid lesions suggests that the virulence of M. tuberculosis for cattle needs to be readdressed. We used an experimental bovine infection model to test the virulence of well-characterized strains of M. tuberculosis and M. bovis in cattle, choosing the genome-sequenced strains M. tuberculosis H37Rv and M. bovis 2122/97. Cattle were infected with approximately 10(6) CFU of M. tuberculosis H37Rv or M. bovis 2122/97, and sacrificed 17 weeks post-infection. IFN-gamma and tuberculin skin tests indicated that both M. bovis 2122 and M. tuberculosis H37Rv were equally infective and triggered strong cell-mediated immune responses, albeit with some indication of differential antigen-specific responses. Postmortem examination revealed that while M. bovis 2122/97-infected animals all showed clear pathology indicative of bovine tuberculosis, the M. tuberculosis-infected animals showed no pathology. Culturing of infected tissues revealed that M. tuberculosis was able to persist in the majority of animals, albeit at relatively low bacillary loads. In revisiting the early work on host preference across the M. tuberculosis complex, we have shown M. tuberculosis H37Rv is avirulent for cattle, and propose that the immune status of the animal, or genotype of the infecting bacillus, may have significant bearing on the virulence of a strain for cattle. This work will serve as a baseline for future studies into the genetic basis of host preference, and in particular the molecular basis of virulence in M. bovis.
Conflict of interest statement
Figures
References
-
- Cole ST, Brosch R, Parkhill J, Garnier T, Churcher C, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. - PubMed
-
- Smith NH, Kremer K, Inwald JJ, Dale J, Driscoll JR, et al. Ecotypes of the Mycobacterium tuberculosis complex. J Theo Biol. 2006;239:220–225. - PubMed
-
- Smith T. Two varieties of the tubercle bacillus from mammals. Trans Assoc Am Physicians. 1896;11:75–95.
-
- von Behring E. Serum Therapy in Therapeutics and Medical Science. Nobel Lectures, Physiology or Medicine 1901-1921: World Scientific Publishing Company. 1901.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

