Diacylglycerol-stimulated endocytosis of transferrin in trypanosomatids is dependent on tyrosine kinase activity
- PMID: 20049089
- PMCID: PMC2796386
- DOI: 10.1371/journal.pone.0008538
Diacylglycerol-stimulated endocytosis of transferrin in trypanosomatids is dependent on tyrosine kinase activity
Abstract
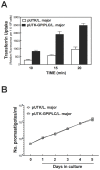
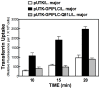
Small molecule regulation of cell function is an understudied area of trypanosomatid biology. In Trypanosoma brucei diacylglycerol (DAG) stimulates endocytosis of transferrin (Tf). However, it is not known whether other trypanosomatidae respond similarly to the lipid. Further, the biochemical pathways involved in DAG signaling to the endocytic system in T. brucei are unknown, as the parasite genome does not encode canonical DAG receptors (e.g. C1-domains). We established that DAG stimulates endocytosis of Tf in Leishmania major, and we evaluated possible effector enzymes in the pathway with multiple approaches. First, a heterologously expressed glycosylphosphatidylinositol phospholipase C (GPI-PLC) activated endocytosis of Tf 300% in L. major. Second, exogenous phorbol ester and DAGs promoted Tf endocytosis in L. major. In search of possible effectors of DAG signaling, we discovered a novel C1-like domain (i.e. C1_5) in trypanosomatids, and we identified protein Tyr kinases (PTKs) linked with C1_5 domains in T. brucei, T. cruzi, and L. major. Consequently, we hypothesized that trypanosome PTKs might be effector enzymes for DAG signaling. General uptake of Tf was reduced by inhibitors of either Ser/Thr or Tyr kinases. However, DAG-stimulated endocytosis of Tf was blocked only by an inhibitor of PTKs, in both T. brucei and L. major. We conclude that (i) DAG activates Tf endocytosis in L. major, and that (ii) PTKs are effectors of DAG-stimulated endocytosis of Tf in trypanosomatids. DAG-stimulated endocytosis of Tf may be a T. brucei adaptation to compete effectively with host cells for vertebrate Tf in blood, since DAG does not enhance endocytosis of Tf in human cells.
Conflict of interest statement
Figures



Similar articles
-
Glycosylphosphatidylinositol-specific phospholipase C regulates transferrin endocytosis in the African trypanosome.Biochem J. 2009 Feb 1;417(3):685-94. doi: 10.1042/BJ20080167. Biochem J. 2009. PMID: 18785878
-
Glycogen Synthase Kinase 3β Promotes the Endocytosis of Transferrin in the African Trypanosome.ACS Infect Dis. 2016 Jul 8;2(7):518-28. doi: 10.1021/acsinfecdis.6b00077. Epub 2016 Jun 16. ACS Infect Dis. 2016. PMID: 27626104 Free PMC article.
-
Pseudokinase NRP1 facilitates endocytosis of transferrin in the African trypanosome.Sci Rep. 2022 Nov 3;12(1):18572. doi: 10.1038/s41598-022-22054-x. Sci Rep. 2022. PMID: 36329148 Free PMC article.
-
C1 domains exposed: from diacylglycerol binding to protein-protein interactions.Biochim Biophys Acta. 2006 Aug;1761(8):827-37. doi: 10.1016/j.bbalip.2006.05.001. Epub 2006 May 13. Biochim Biophys Acta. 2006. PMID: 16861033 Review.
-
Signal transduction in vascular smooth muscle: diacylglycerol second messengers and PKC action.Am J Physiol. 1994 Sep;267(3 Pt 1):C659-78. doi: 10.1152/ajpcell.1994.267.3.C659. Am J Physiol. 1994. PMID: 7943196 Review.
Cited by
-
The Trypanosomal Transferrin Receptor of Trypanosoma Brucei-A Review.Trop Med Infect Dis. 2019 Oct 1;4(4):126. doi: 10.3390/tropicalmed4040126. Trop Med Infect Dis. 2019. PMID: 31581506 Free PMC article. Review.
-
New chemical scaffolds for human african trypanosomiasis lead discovery from a screen of tyrosine kinase inhibitor drugs.Antimicrob Agents Chemother. 2014;58(4):2202-10. doi: 10.1128/AAC.01691-13. Epub 2014 Jan 27. Antimicrob Agents Chemother. 2014. PMID: 24468788 Free PMC article.
-
Iron Homeostasis and Trypanosoma brucei Associated Immunopathogenicity Development: A Battle/Quest for Iron.Biomed Res Int. 2015;2015:819389. doi: 10.1155/2015/819389. Epub 2015 May 18. Biomed Res Int. 2015. PMID: 26090446 Free PMC article. Review.
-
Protozoan phagotrophy from predators to parasites: An overview of the enigmatic cytostome-cytopharynx complex of Trypanosoma cruzi.J Eukaryot Microbiol. 2022 Nov;69(6):e12896. doi: 10.1111/jeu.12896. Epub 2022 Mar 8. J Eukaryot Microbiol. 2022. PMID: 35175673 Free PMC article. Review.
-
Novel aspects of iron homeostasis in pathogenic bloodstream form Trypanosoma brucei.PLoS Pathog. 2021 Jun 23;17(6):e1009696. doi: 10.1371/journal.ppat.1009696. eCollection 2021 Jun. PLoS Pathog. 2021. PMID: 34161395 Free PMC article.
References
-
- Sorkin A, Von Zastrow M. Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol. 2002;3:600–614. - PubMed
-
- Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein-protein interactions. Biochim Biophys Acta. 2006;1761:827–837. - PubMed
-
- Hereld D, Krakow JL, Bangs JD, Hart GW, Englund PT. A phospholipase C from Trypanosoma brucei which selectively cleaves the glycolipid on the variant surface glycoprotein. J Biol Chem. 1986;261:13813–13819. - PubMed
-
- Fox JA, Duszenko M, Ferguson MA, Low MG, Cross GAM. Purification and characterization of a novel glycan-phosphatidylinositol-specific phospholipase C from Trypanosoma brucei. J Biol Chem. 1986;261:15767–15771. - PubMed
-
- Morris JC, Ping-Sheng L, Shen TY, Mensa-Wilmot K. Glycan requirements of glycosylphosphatidylinositol phospholipase C from Trypanosoma brucei. Glucosaminylinositol derivatives inhibit phosphatidylinositol phospholipase C. J Biol Chem. 1995;270:2517–2524. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

