Does rapid metabolism ensure negligible risk from bisphenol A?
- PMID: 20049111
- PMCID: PMC2801165
- DOI: 10.1289/ehp.0901010
Does rapid metabolism ensure negligible risk from bisphenol A?
Abstract
Background: Bisphenol A (BPA) risks are being evaluated by many regulatory bodies because exposure is widespread and the potential exists for toxicity at low doses.
Objective: We evaluated evidence that BPA is cleared more rapidly in humans than in rats in relation to BPA risk assessment.
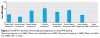
Discussion: The European Food Safety Authority (EFSA) relied on pharmacokinetic evidence to conclude that rodent toxicity data are not directly relevant to human risk assessment. Further, the EFSA argues that rapid metabolism will result in negligible exposure during the perinatal period because of BPA glucuronidation in pregnant women or sulfation in newborns. These arguments fail to consider the deconjugation of BPA glucuronide in utero by beta-glucuronidase, an enzyme that is present in high concentrations in placenta and various other tissues. Further, arylsulfatase C, which reactivates endogenous sulfated estrogens, develops early in life and so may deconjugate BPA sulfate in newborns. Biomonitoring studies and laboratory experiments document free BPA in rat and human maternal, placental, and fetal tissues, indicating that human BPA exposure is not negligible. The pattern of these detections is consistent with deconjugation in the placenta, resulting in fetal exposure. The tolerable daily intake set by the EFSA (0.05 mg/kg/day) is well above effect levels reported in some animal studies.
Conclusion: This potential risk should not be dismissed on the basis of an uncertain pharmacokinetic argument. Rather, risk assessors need to decipher the BPA dose response and apply it to humans with comprehensive pharmacokinetic models that account for metabolite deconjugation.
Keywords: bisphenol A; endocrine disruption; fetus; glucuronidation; metabolism; neonate; β-glucuronidase.
Figures
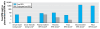
Similar articles
-
Critical evaluation of key evidence on the human health hazards of exposure to bisphenol A.Crit Rev Toxicol. 2011 Apr;41(4):263-91. doi: 10.3109/10408444.2011.558487. Crit Rev Toxicol. 2011. PMID: 21438738 Free PMC article. Review.
-
Conjugation and deconjugation reactions within the fetoplacental compartment in a sheep model: a key factor determining bisphenol A fetal exposure.Drug Metab Dispos. 2015 Apr;43(4):467-76. doi: 10.1124/dmd.114.061291. Epub 2015 Jan 9. Drug Metab Dispos. 2015. PMID: 25576162
-
Bisphenol A glucuronide deconjugation is a determining factor of fetal exposure to bisphenol A.Environ Int. 2016 Jan;86:52-9. doi: 10.1016/j.envint.2015.10.006. Epub 2015 Oct 24. Environ Int. 2016. PMID: 26540084
-
Integrated exposure and risk characterization of bisphenol-A in Europe.Food Chem Toxicol. 2016 Dec;98(Pt B):134-147. doi: 10.1016/j.fct.2016.10.017. Epub 2016 Oct 18. Food Chem Toxicol. 2016. PMID: 27769850
-
Derivation of a bisphenol A oral reference dose (RfD) and drinking-water equivalent concentration.J Toxicol Environ Health B Crit Rev. 2008 Feb;11(2):69-146. doi: 10.1080/10937400701724303. J Toxicol Environ Health B Crit Rev. 2008. PMID: 18188738 Review.
Cited by
-
Novel insights into the role of bisphenol A (BPA) in genomic instability.NAR Cancer. 2024 Sep 24;6(3):zcae038. doi: 10.1093/narcan/zcae038. eCollection 2024 Sep. NAR Cancer. 2024. PMID: 39319028 Free PMC article. Review.
-
Placental profiling of UGT1A enzyme expression and activity and interactions with preeclampsia at term.Eur J Drug Metab Pharmacokinet. 2015 Dec;40(4):471-80. doi: 10.1007/s13318-014-0243-4. Epub 2014 Dec 3. Eur J Drug Metab Pharmacokinet. 2015. PMID: 25465229 Free PMC article.
-
Influence of Bisphenol A on Type 2 Diabetes Mellitus.Int J Environ Res Public Health. 2016 Oct 6;13(10):989. doi: 10.3390/ijerph13100989. Int J Environ Res Public Health. 2016. PMID: 27782064 Free PMC article. Review.
-
Bisphenol A: an emerging threat to female fertility.Reprod Biol Endocrinol. 2020 Mar 14;18(1):22. doi: 10.1186/s12958-019-0558-8. Reprod Biol Endocrinol. 2020. PMID: 32171313 Free PMC article. Review.
-
Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A.Environ Health Perspect. 2010 Aug;118(8):1055-70. doi: 10.1289/ehp.0901716. Epub 2010 Mar 23. Environ Health Perspect. 2010. PMID: 20338858 Free PMC article. Review.
References
-
- Allegaert K, de Hoon J, Verbesselt R, Vanhole C, Devlieger H, Tibboel D. Intra- and interindividual variability of glucuronidation of paracetamol during repeated administration of propacetamol in neonates. Acta Paediatr. 2005;94:1273–1279. - PubMed
-
- Bohnenstengel F, Kroemer HK, Sperker B. In vitro cleavage of paracetamol glucuronide by human liver and kidney beta-glucuronidase: determination of paracetamol by capillary electrophoresis. J Chromatog B Biomed Sci Appl. 1999;721:295–299. - PubMed
-
- Brunelle FM, Verbeeck RK. Conjugation-deconjugation cycling of diflunisal via beta-glucuronidase catalyzed hydrolysis of its acyl glucuronide in the rat. Life Sci. 1997;60:2013–2021. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

