Mammary Fat Can Adjust Prolactin Effect on Mammary Epithelial Cells via Leptin and Estrogen
- PMID: 20049155
- PMCID: PMC2798110
- DOI: 10.1155/2009/427260
Mammary Fat Can Adjust Prolactin Effect on Mammary Epithelial Cells via Leptin and Estrogen
Abstract
Leptin, like estrogen, is one of the endo/paracrine factors, which are synthesized in and secreted from mature adipocytes. The roles of the mammary fat pad and mammary adipocytes in the initiation of lactation are not clear. In this study, we showed that combination of prolactin, leptin and estrogen elevated the expression of the milk protein beta-lactoglobulin. We also showed that after prolactin stimulate the secretion of leptin from the mammary fat, leptin upregulated the expression of estrogen receptor alpha in the mammary epithelial cells. Also, prolactin affected aromatase mRNA expression in the bovine mammary fat and we demonstrated that leptin and prolactin can affect cholesterol secretion from explants in culture to the medium. Therefore, we suggest that prolactin initiates estrogen expression (as represented by aromatase mRNA) in the mammary fat pad, whereas leptin stimulates estrogen receptor alpha expression in the mammary epithelial cells. We hypothesize that leptin and estrogen, secreted from the mammary fat regulate lactation after stimulation of prolactin.
Figures

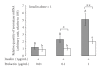
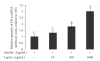

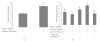
Similar articles
-
TRIENNIAL LACTATION SYMPOSIUM/BOLFA: Adipokines affect mammary growth and function in farm animals.J Anim Sci. 2017 Dec;95(12):5689-5700. doi: 10.2527/jas2017.1777. J Anim Sci. 2017. PMID: 29293788 Free PMC article. Review.
-
Prolactin affects leptin action in the bovine mammary gland via the mammary fat pad.J Endocrinol. 2006 Nov;191(2):407-13. doi: 10.1677/joe.1.06913. J Endocrinol. 2006. PMID: 17088410
-
Leptin affects prolactin action on milk protein and fat synthesis in the bovine mammary gland.J Dairy Sci. 2004 Sep;87(9):2941-6. doi: 10.3168/jds.S0022-0302(04)73425-6. J Dairy Sci. 2004. PMID: 15375055
-
Growth hormone and lactogenic hormones can reduce the leptin mRNA expression in bovine mammary epithelial cells.Domest Anim Endocrinol. 2006 Jul;31(1):88-96. doi: 10.1016/j.domaniend.2005.09.002. Epub 2005 Sep 21. Domest Anim Endocrinol. 2006. PMID: 16198527
-
Regulation of gene expression in the bovine mammary gland by ovarian steroids.J Dairy Sci. 2007 Jun;90 Suppl 1:E55-65. doi: 10.3168/jds.2006-466. J Dairy Sci. 2007. PMID: 17517752 Review.
Cited by
-
Association of bovine leptin polymorphisms with energy output and energy storage traits in progeny tested Holstein-Friesian dairy cattle sires.BMC Genet. 2010 Jul 29;11:73. doi: 10.1186/1471-2156-11-73. BMC Genet. 2010. PMID: 20670403 Free PMC article.
-
TRIENNIAL LACTATION SYMPOSIUM/BOLFA: Adipokines affect mammary growth and function in farm animals.J Anim Sci. 2017 Dec;95(12):5689-5700. doi: 10.2527/jas2017.1777. J Anim Sci. 2017. PMID: 29293788 Free PMC article. Review.
-
Essential Role for Zinc Transporter 2 (ZnT2)-mediated Zinc Transport in Mammary Gland Development and Function during Lactation.J Biol Chem. 2015 May 22;290(21):13064-78. doi: 10.1074/jbc.M115.637439. Epub 2015 Apr 7. J Biol Chem. 2015. PMID: 25851903 Free PMC article.
-
An "elite hacker": breast tumors exploit the normal microenvironment program to instruct their progression and biological diversity.Cell Adh Migr. 2012 May-Jun;6(3):236-48. doi: 10.4161/cam.20880. Epub 2012 May 1. Cell Adh Migr. 2012. PMID: 22863741 Free PMC article. Review.
-
Proteomic analysis of the nuclear phosphorylated proteins in dairy cow mammary epithelial cells treated with estrogen.In Vitro Cell Dev Biol Anim. 2012 Aug;48(7):449-57. doi: 10.1007/s11626-012-9531-y. Epub 2012 Jul 18. In Vitro Cell Dev Biol Anim. 2012. PMID: 22806971
References
-
- Schäffler A, Müller-Ladner U, Schölmerich J, Büchler C. Role of adipose tissue as an inflammatory organ in human diseases. Endocrine Reviews. 2006;27(5):449–467. - PubMed
-
- Walden PD, Ruan W, Feldman M, Kleinberg DL. Evidence that the mammary fat pad mediates the action of growth hormone in mammary gland development. Endocrinology. 1998;139(2):659–662. - PubMed
-
- Li RW, Meyer MJ, van Tassell CP, et al. Identification of estrogen-responsive genes in the parenchyma and fat pad of the bovine mammary gland by microarray analysis. Physiological Genomics. 2006;27(1):42–53. - PubMed
-
- Meyer MJ, Capuco AV, Boisclair YR, van Amburgh ME. Estrogen-dependent responses of the mammary fat pad in prepubertal dairy heifers. Journal of Endocrinology. 2006;190(3):819–827. - PubMed
-
- Zangani D, et al. Adipocyte-epithelial interactions regulate the in vitro development of normal mammary epithelial cells. Experimental Cell Research. 1999;247(2):399–409. - PubMed
LinkOut - more resources
Full Text Sources

