Nanotechnology and in situ remediation: a review of the benefits and potential risks
- PMID: 20049198
- PMCID: PMC2799454
- DOI: 10.1289/ehp.0900793
Nanotechnology and in situ remediation: a review of the benefits and potential risks
Abstract
Objective: Although industrial sectors involving semiconductors; memory and storage technologies; display, optical, and photonic technologies; energy; biotechnology; and health care produce the most products that contain nanomaterials, nanotechnology is also used as an environmental technology to protect the environment through pollution prevention, treatment, and cleanup. In this review, we focus on environmental cleanup and provide a background and overview of current practice; research findings; societal issues; potential environment, health, and safety implications; and future directions for nanoremediation. We do not present an exhaustive review of chemistry/engineering methods of the technology but rather an introduction and summary of the applications of nanotechnology in remediation. We also discuss nanoscale zerovalent iron in detail.
Data sources: We searched the Web of Science for research studies and accessed recent publicly available reports from the U.S. Environmental Protection Agency and other agencies and organizations that addressed the applications and implications associated with nanoremediation techniques. We also conducted personal interviews with practitioners about specific site remediations.
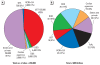
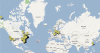
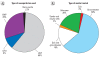
Data synthesis: We aggregated information from 45 sites, a representative portion of the total projects under way, to show nanomaterials used, types of pollutants addressed, and organizations responsible for each site.
Conclusions: Nanoremediation has the potential not only to reduce the overall costs of cleaning up large-scale contaminated sites but also to reduce cleanup time, eliminate the need for treatment and disposal of contaminated soil, and reduce some contaminant concentrations to near zero-all in situ. Proper evaluation of nanoremediation, particularly full-scale ecosystem-wide studies, needs to be conducted to prevent any potential adverse environmental impacts.
Keywords: environmental implications; environmental technology; hazardous wastes; nano-remediation; nanotechnology; pollutants; remediation; toxicity; waste sites; zerovalent iron.
Figures
Republished in
-
Nanotechnology and in situ remediation: a review of the benefits and potential risks.Cien Saude Colet. 2011 Jan;16(1):165-78. doi: 10.1590/s1413-81232011000100020. Cien Saude Colet. 2011. PMID: 21180825
Comment in
-
Hit or miss?: benefits and risks of using nanoparticles for in situ remediation.Environ Health Perspect. 2009 Dec;117(12):A552. doi: 10.1289/ehp.117-a552a. Environ Health Perspect. 2009. PMID: 20049191 Free PMC article. No abstract available.
References
-
- Adams LK, Lyon DY, Alvarez PJJ. Comparative ecotoxicity of nanoscale TiO2, SiO2 and ZnO water suspensions. Water Res. 2006;40:3527–3532. - PubMed
-
- American Recovery and Reinvestment Act. 2009. [[accessed 20 October 2009]]. Public Law 111‐5. Available: http://frwebgate.access.gpo.gov/cgi-bin/getdoc.cgi?dbname=111_cong_bills....
-
- Auffan M, Decome L, Rose J, Orsiere T, De Meo M, Briois V, et al. In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblast: a physicochemical and cytogenotoxical study. Environ Sci Technol. 2006;40(14):4367–4373. - PubMed
-
- Biswas P, Wu C-Y. Nanoparticles and the environment. J Air Waste Manag. 2005;55:708–746. - PubMed
-
- Boxall ABA, Tiede K, Chaudhry Q. Engineered nanomaterials in soils and water: how do they behave and could they pose a risk to human health? Nanomedicine. 2007;2(6):919–927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

