Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system
- PMID: 20049207
- PMCID: PMC2799462
- DOI: 10.1289/ehp.0900923
Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system
Abstract
Background: Developmental exposure to environmental estrogens is associated with adverse consequences later in life. Exposure to genistin (GIN), the glycosylated form of the phytoestrogen genistein (GEN) found in soy products, is of concern because approximately 20% of U.S. infants are fed soy formula. High circulating levels of GEN have been measured in the serum of these infants, indicating that GIN is readily absorbed, hydrolyzed, and circulated.
Objectives: We investigated whether orally administered GIN is estrogenic in neonatal mice and whether it causes adverse effects on the developing female reproductive tract.
Methods: Female CD-1 mice were treated on postnatal days 1-5 with oral GIN (6.25, 12.5, 25, or 37.5 mg/kg/day; GEN-equivalent doses), oral GEN (25, 37.5, or 75 mg/kg/day), or subcutaneous GEN (12.5, 20, or 25 mg/kg/day). Estrogenic activity was measured on day 5 by determining uterine wet weight gain and induction of the estrogen-responsive gene lactoferrin. Vaginal opening, estrous cyclicity, fertility, and morphologic alterations in the ovary/reproductive tract were examined.
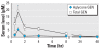
Results: Oral GIN elicited an estrogenic response in the neonatal uterus, whereas the response to oral GEN was much weaker. Oral GIN altered ovarian differentiation (i.e., multioocyte follicles), delayed vaginal opening, caused abnormal estrous cycles, decreased fertility, and delayed parturition.
Conclusions: Our results support the idea that the dose of the physiologically active compound reaching the target tissue, rather than the administered dose or route, is most important in modeling chemical exposures. This is particularly true with young animals in which phase II metabolism capacity is underdeveloped relative to adults.
Keywords: development; diethylstilbestrol; endocrine disruptors; environmental estrogen; isoflavone; ovary.
Figures

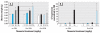
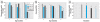

Similar articles
-
Circulating levels of genistein in the neonate, apart from dose and route, predict future adverse female reproductive outcomes.Reprod Toxicol. 2011 Apr;31(3):272-9. doi: 10.1016/j.reprotox.2010.10.001. Epub 2010 Oct 15. Reprod Toxicol. 2011. PMID: 20955782 Free PMC article. Review.
-
Acute and chronic effects of oral genistein administration in neonatal mice.Biol Reprod. 2010 Jul;83(1):114-21. doi: 10.1095/biolreprod.109.080549. Epub 2010 Mar 31. Biol Reprod. 2010. PMID: 20357267 Free PMC article.
-
Multigenerational reproductive study of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study).Natl Toxicol Program Tech Rep Ser. 2008 Mar;(539):1-266. Natl Toxicol Program Tech Rep Ser. 2008. PMID: 18685713
-
Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring.Reprod Toxicol. 2004 Aug-Sep;18(6):803-11. doi: 10.1016/j.reprotox.2004.05.002. Reprod Toxicol. 2004. PMID: 15279878
-
Disruption of the developing female reproductive system by phytoestrogens: genistein as an example.Mol Nutr Food Res. 2007 Jul;51(7):832-44. doi: 10.1002/mnfr.200600258. Mol Nutr Food Res. 2007. PMID: 17604387 Review.
Cited by
-
Soy but not bisphenol A (BPA) induces hallmarks of polycystic ovary syndrome (PCOS) and related metabolic co-morbidities in rats.Reprod Toxicol. 2014 Nov;49:209-18. doi: 10.1016/j.reprotox.2014.09.003. Epub 2014 Sep 19. Reprod Toxicol. 2014. PMID: 25242113 Free PMC article.
-
Endocrine disruption of brain sexual differentiation by developmental PCB exposure.Endocrinology. 2011 Feb;152(2):581-94. doi: 10.1210/en.2010-1103. Epub 2010 Dec 29. Endocrinology. 2011. PMID: 21190954 Free PMC article.
-
State of the evidence 2017: an update on the connection between breast cancer and the environment.Environ Health. 2017 Sep 2;16(1):94. doi: 10.1186/s12940-017-0287-4. Environ Health. 2017. PMID: 28865460 Free PMC article. Review.
-
The health effects of soy: A reference guide for health professionals.Front Nutr. 2022 Aug 11;9:970364. doi: 10.3389/fnut.2022.970364. eCollection 2022. Front Nutr. 2022. PMID: 36034914 Free PMC article. Review.
-
Permanent oviduct posteriorization after neonatal exposure to the phytoestrogen genistein.Environ Health Perspect. 2011 Nov;119(11):1575-82. doi: 10.1289/ehp.1104018. Epub 2011 Aug 2. Environ Health Perspect. 2011. PMID: 21810550 Free PMC article.
References
-
- Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180(3 Pt 1):737–743. - PubMed
-
- Bhatia J, Greer F. Use of soy protein-based formulas in infant feeding. Pediatrics. 2008;121(5):1062–1068. - PubMed
-
- Champlin AK, Dorr DL, Gates AH. Determining the stage of the estrous cycle in the mouse by the appearance of the vagina. Biol Reprod. 1973;8(4):491–494. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

