Loss of Smyhc1 or Hsp90alpha1 function results in different effects on myofibril organization in skeletal muscles of zebrafish embryos
- PMID: 20049323
- PMCID: PMC2797074
- DOI: 10.1371/journal.pone.0008416
Loss of Smyhc1 or Hsp90alpha1 function results in different effects on myofibril organization in skeletal muscles of zebrafish embryos
Abstract
Background: Myofibrillogenesis requires the correct folding and assembly of sarcomeric proteins into highly organized sarcomeres. Heat shock protein 90alpha1 (Hsp90alpha1) has been implicated as a myosin chaperone that plays a key role in myofibrillogenesis. Knockdown or mutation of hsp90alpha1 resulted in complete disorganization of thick and thin filaments and M- and Z-line structures. It is not clear whether the disorganization of these sarcomeric structures is due to a direct effect from loss of Hsp90alpha1 function or indirectly through the disorganization of myosin thick filaments.
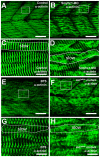
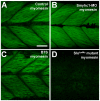
Methodology/principal findings: In this study, we carried out a loss-of-function analysis of myosin thick filaments via gene-specific knockdown or using a myosin ATPase inhibitor BTS (N-benzyl-p-toluene sulphonamide) in zebrafish embryos. We demonstrated that knockdown of myosin heavy chain 1 (myhc1) resulted in sarcomeric defects in the thick and thin filaments and defective alignment of Z-lines. Similarly, treating zebrafish embryos with BTS disrupted thick and thin filament organization, with little effect on the M- and Z-lines. In contrast, loss of Hsp90alpha1 function completely disrupted all sarcomeric structures including both thick and thin filaments as well as the M- and Z-lines.
Conclusion/significance: Together, these studies indicate that the hsp90alpha1 mutant phenotype is not simply due to disruption of myosin folding and assembly, suggesting that Hsp90alpha1 may play a role in the assembly and organization of other sarcomeric structures.
Conflict of interest statement
Figures




Similar articles
-
Knockdown and overexpression of Unc-45b result in defective myofibril organization in skeletal muscles of zebrafish embryos.BMC Cell Biol. 2010 Sep 17;11:70. doi: 10.1186/1471-2121-11-70. BMC Cell Biol. 2010. PMID: 20849610 Free PMC article.
-
Heat-shock protein 90alpha1 is required for organized myofibril assembly in skeletal muscles of zebrafish embryos.Proc Natl Acad Sci U S A. 2008 Jan 15;105(2):554-9. doi: 10.1073/pnas.0707330105. Epub 2008 Jan 8. Proc Natl Acad Sci U S A. 2008. PMID: 18182494 Free PMC article.
-
The Effects of Hsp90α1 Mutations on Myosin Thick Filament Organization.PLoS One. 2015 Nov 12;10(11):e0142573. doi: 10.1371/journal.pone.0142573. eCollection 2015. PLoS One. 2015. PMID: 26562659 Free PMC article.
-
The vertebrate myosin heavy chain: genetics and assembly properties.Cell Struct Funct. 1997 Feb;22(1):123-9. doi: 10.1247/csf.22.123. Cell Struct Funct. 1997. PMID: 9113398 Review.
-
Determining structure/function relationships for sarcomeric myosin heavy chain by genetic and transgenic manipulation of Drosophila.Microsc Res Tech. 2000 Sep 15;50(6):430-42. doi: 10.1002/1097-0029(20000915)50:6<430::AID-JEMT2>3.0.CO;2-E. Microsc Res Tech. 2000. PMID: 10998634 Review.
Cited by
-
Functional analysis of slow myosin heavy chain 1 and myomesin-3 in sarcomere organization in zebrafish embryonic slow muscles.J Genet Genomics. 2012 Feb;39(2):69-80. doi: 10.1016/j.jgg.2012.01.005. Epub 2012 Jan 21. J Genet Genomics. 2012. PMID: 22361506 Free PMC article.
-
Structural analysis of alterations in zebrafish muscle differentiation induced by simvastatin and their recovery with cholesterol.J Histochem Cytochem. 2015 Jun;63(6):427-37. doi: 10.1369/0022155415580396. Epub 2015 Mar 18. J Histochem Cytochem. 2015. PMID: 25786435 Free PMC article.
-
Phasor approach of Mueller matrix optical scanning microscopy for biological tissue imaging.Biophys J. 2021 Aug 3;120(15):3112-3125. doi: 10.1016/j.bpj.2021.06.008. Epub 2021 Jul 3. Biophys J. 2021. PMID: 34224693 Free PMC article.
-
Identification of novel MYO18A interaction partners required for myoblast adhesion and muscle integrity.Sci Rep. 2016 Nov 8;6:36768. doi: 10.1038/srep36768. Sci Rep. 2016. PMID: 27824130 Free PMC article.
-
Smyd1b is required for skeletal and cardiac muscle function in zebrafish.Mol Biol Cell. 2013 Nov;24(22):3511-21. doi: 10.1091/mbc.E13-06-0352. Epub 2013 Sep 25. Mol Biol Cell. 2013. PMID: 24068325 Free PMC article.
References
-
- Agarkova I, Perriard JC. The M-band: an elastic web that crosslinks thick filaments in the center of the sarcomere. Trends Cell Biol. 2005;15:477–485. - PubMed
-
- Boateng SY, Goldspink PH. Assembly and maintenance of the sarcomere night and day. Cardiovasc Res. 2008;77:667–675. - PubMed
-
- Clark KA, McElhinny AS, Beckerle MC, Gregorio CC. Striated muscle cytoarchitecture: an intricate web of form and function. Annu Rev Cell Dev Biol. 2002;18:637–706. - PubMed
-
- Frank D, Kuhn C, Katus HA, Frey N. The sarcomeric Z-disc: a nodal point in signalling and disease. J Mol Med. 2006;84:446–468. - PubMed
-
- Gregorio CC, Granzier H, Sorimachi H, Labeit S. Muscle assembly: a titanic achievement? Curr Opin Cell Biol. 1999;11:18–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

