Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation
- PMID: 20049328
- PMCID: PMC2797305
- DOI: 10.1371/journal.pone.0008561
Regulation of BMAL1 protein stability and circadian function by GSK3beta-mediated phosphorylation
Abstract
Background: Circadian rhythms govern a large array of physiological and metabolic functions. To achieve plasticity in circadian regulation, proteins constituting the molecular clock machinery undergo various post-translational modifications (PTMs), which influence their activity and intracellular localization. The core clock protein BMAL1 undergoes several PTMs. Here we report that the Akt-GSK3beta signaling pathway regulates BMAL1 protein stability and activity.
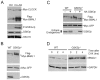
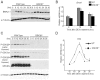
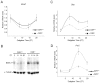
Principal findings: GSK3beta phosphorylates BMAL1 specifically on Ser 17 and Thr 21 and primes it for ubiquitylation. In the absence of GSK3beta-mediated phosphorylation, BMAL1 becomes stabilized and BMAL1 dependent circadian gene expression is dampened. Dopamine D2 receptor mediated signaling, known to control the Akt-GSK3beta pathway, influences BMAL1 stability and in vivo circadian gene expression in striatal neurons.
Conclusions: These findings uncover a previously unknown mechanism of circadian clock control. The GSK3beta kinase phosphorylates BMAL1, an event that controls the stability of the protein and the amplitude of circadian oscillation. BMAL1 phosphorylation appears to be an important regulatory step in maintaining the robustness of the circadian clock.
Conflict of interest statement
Figures


Similar articles
-
PIWIL1 suppresses circadian rhythms through GSK3β-induced phosphorylation and degradation of CLOCK and BMAL1 in cancer cells.J Cell Mol Med. 2019 Jul;23(7):4689-4698. doi: 10.1111/jcmm.14377. Epub 2019 May 16. J Cell Mol Med. 2019. PMID: 31099187 Free PMC article.
-
A serine cluster mediates BMAL1-dependent CLOCK phosphorylation and degradation.Cell Cycle. 2009 Dec 15;8(24):4138-46. doi: 10.4161/cc.8.24.10273. Epub 2009 Dec 8. Cell Cycle. 2009. PMID: 19946213 Free PMC article.
-
Circadian rhythmicity of active GSK3 isoforms modulates molecular clock gene rhythms in the suprachiasmatic nucleus.J Biol Rhythms. 2015 Apr;30(2):155-60. doi: 10.1177/0748730415573167. Epub 2015 Feb 27. J Biol Rhythms. 2015. PMID: 25724980 Free PMC article.
-
Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues.Neurochem Int. 2018 Oct;119:11-16. doi: 10.1016/j.neuint.2017.12.013. Epub 2018 Jan 3. Neurochem Int. 2018. PMID: 29305918 Review.
-
Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock.Biomedicines. 2025 Mar 13;13(3):705. doi: 10.3390/biomedicines13030705. Biomedicines. 2025. PMID: 40149680 Free PMC article. Review.
Cited by
-
At the Interface of Lifestyle, Behavior, and Circadian Rhythms: Metabolic Implications.Front Nutr. 2019 Aug 28;6:132. doi: 10.3389/fnut.2019.00132. eCollection 2019. Front Nutr. 2019. PMID: 31555652 Free PMC article. Review.
-
Circadian clocks, cognition, and Alzheimer's disease: synaptic mechanisms, signaling effectors, and chronotherapeutics.Mol Neurodegener. 2022 May 7;17(1):35. doi: 10.1186/s13024-022-00537-9. Mol Neurodegener. 2022. PMID: 35525980 Free PMC article. Review.
-
Mathematical analysis of robustness of oscillations in models of the mammalian circadian clock.PLoS Comput Biol. 2022 Mar 18;18(3):e1008340. doi: 10.1371/journal.pcbi.1008340. eCollection 2022 Mar. PLoS Comput Biol. 2022. PMID: 35302984 Free PMC article.
-
Circadian rhythms, the molecular clock, and skeletal muscle.Curr Top Dev Biol. 2011;96:231-71. doi: 10.1016/B978-0-12-385940-2.00009-7. Curr Top Dev Biol. 2011. PMID: 21621073 Free PMC article. Review.
-
Emerging role of circadian clock disruption in alcohol-induced liver disease.Am J Physiol Gastrointest Liver Physiol. 2018 Sep 1;315(3):G364-G373. doi: 10.1152/ajpgi.00010.2018. Epub 2018 May 31. Am J Physiol Gastrointest Liver Physiol. 2018. PMID: 29848023 Free PMC article. Review.
References
-
- Sahar S, Sassone-Corsi P. Metabolism and cancer: the circadian clock connection. Nat Rev Cancer. 2009;9:886–896. - PubMed
-
- Wijnen H, Young MW. Interplay of circadian clocks and metabolic rhythms. Annu Rev Genet. 2006;40:409–448. - PubMed
-
- Gallego M, Virshup DM. Post-translational modifications regulate the ticking of the circadian clock. Nat Rev Mol Cell Biol. 2007;8:139–148. - PubMed
-
- Sanada K, Okano T, Fukada Y. Mitogen-activated protein kinase phosphorylates and negatively regulates basic helix-loop-helix-PAS transcription factor BMAL1. J Biol Chem. 2002;277:267–271. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

