Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth
- PMID: 20049329
- PMCID: PMC2797307
- DOI: 10.1371/journal.pone.0008551
Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth
Abstract
Background: The most well known reproductive consequence of residence at high altitude (HA >2700 m) is reduction in fetal growth. Reduced fetoplacental oxygenation is an underlying cause of pregnancy pathologies, including intrauterine growth restriction and preeclampsia, which are more common at HA. Therefore, altitude is a natural experimental model to study the etiology of pregnancy pathophysiologies. We have shown that the proximate cause of decreased fetal growth is not reduced oxygen availability, delivery, or consumption. We therefore asked whether glucose, the primary substrate for fetal growth, might be decreased and/or whether altered fetoplacental glucose metabolism might account for reduced fetal growth at HA.
Methods: Doppler and ultrasound were used to measure maternal uterine and fetal umbilical blood flows in 69 and 58 residents of 400 vs 3600 m. Arterial and venous blood samples from mother and fetus were collected at elective cesarean delivery and analyzed for glucose, lactate and insulin. Maternal delivery and fetal uptakes for oxygen and glucose were calculated.
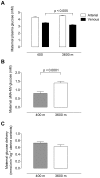
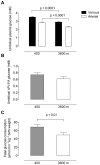
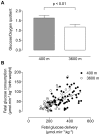
Principal findings: The maternal arterial - venous glucose concentration difference was greater at HA. However, umbilical venous and arterial glucose concentrations were markedly decreased, resulting in lower glucose delivery at 3600 m. Fetal glucose consumption was reduced by >28%, but strongly correlated with glucose delivery, highlighting the relevance of glucose concentration to fetal uptake. At altitude, fetal lactate levels were increased, insulin concentrations decreased, and the expression of GLUT1 glucose transporter protein in the placental basal membrane was reduced.
Conclusion/significance: Our results support that preferential anaerobic consumption of glucose by the placenta at high altitude spares oxygen for fetal use, but limits glucose availability for fetal growth. Thus reduced fetal growth at high altitude is associated with fetal hypoglycemia, hypoinsulinemia and a trend towards lactacidemia. Our data support that placentally-mediated reduction in glucose transport is an initiating factor for reduced fetal growth under conditions of chronic hypoxemia.
Conflict of interest statement
Figures

Similar articles
-
Placental metabolic reprogramming: do changes in the mix of energy-generating substrates modulate fetal growth?Int J Dev Biol. 2010;54(2-3):409-19. doi: 10.1387/ijdb.082798ni. Int J Dev Biol. 2010. PMID: 19924633 Free PMC article. Review.
-
The hemodynamics of late-onset intrauterine growth restriction by MRI.Am J Obstet Gynecol. 2016 Mar;214(3):367.e1-367.e17. doi: 10.1016/j.ajog.2015.10.004. Epub 2015 Oct 22. Am J Obstet Gynecol. 2016. PMID: 26475425
-
Uterine artery blood flow, fetal hypoxia and fetal growth.Philos Trans R Soc Lond B Biol Sci. 2015 Mar 5;370(1663):20140068. doi: 10.1098/rstb.2014.0068. Philos Trans R Soc Lond B Biol Sci. 2015. PMID: 25602072 Free PMC article.
-
Placental glucose transporter 1 and 3 gene expression in Monochorionic twin pregnancies with selective fetal growth restriction.BMC Pregnancy Childbirth. 2021 Mar 27;21(1):260. doi: 10.1186/s12884-021-03744-2. BMC Pregnancy Childbirth. 2021. PMID: 33773574 Free PMC article.
-
Placental Gas Exchange and the Oxygen Supply to the Fetus.Compr Physiol. 2015 Jul 1;5(3):1381-403. doi: 10.1002/cphy.c140073. Compr Physiol. 2015. PMID: 26140722 Review.
Cited by
-
Tracking placental development in health and disease.Nat Rev Endocrinol. 2020 Sep;16(9):479-494. doi: 10.1038/s41574-020-0372-6. Epub 2020 Jun 29. Nat Rev Endocrinol. 2020. PMID: 32601352 Review.
-
Ssc-miR-92b-3p Regulates Porcine Trophoblast Cell Proliferation and Migration via the PFKM Gene.Int J Mol Sci. 2022 Dec 17;23(24):16138. doi: 10.3390/ijms232416138. Int J Mol Sci. 2022. PMID: 36555776 Free PMC article.
-
Hypoxia signaling in human health and diseases: implications and prospects for therapeutics.Signal Transduct Target Ther. 2022 Jul 7;7(1):218. doi: 10.1038/s41392-022-01080-1. Signal Transduct Target Ther. 2022. PMID: 35798726 Free PMC article. Review.
-
Measuring high-altitude adaptation.J Appl Physiol (1985). 2017 Nov 1;123(5):1371-1385. doi: 10.1152/japplphysiol.00321.2017. Epub 2017 Aug 31. J Appl Physiol (1985). 2017. PMID: 28860167 Free PMC article. Review.
-
Differential Expression of Glucose Transporter Proteins GLUT-1, GLUT-3, GLUT-8 and GLUT-12 in the Placenta of Macrosomic, Small-for-Gestational-Age and Growth-Restricted Foetuses.J Clin Med. 2021 Dec 13;10(24):5833. doi: 10.3390/jcm10245833. J Clin Med. 2021. PMID: 34945129 Free PMC article.
References
-
- Hack M, Weissman B, Breslau N, Klein N, Borawski-Clark E, et al. Health of very low birth weight children during their first eight years. J Pediatr. 1993;122:887–892. - PubMed
-
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, et al. Births: final data for 2005. National Vital Statistics Reports. 2007;56:1–103. - PubMed
-
- Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184:946–953. - PubMed
-
- Mongelli M, Gardosi J. Fetal growth. Curr Opin Obstet Gynecol. 2000;12:111–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

