Calcium influx measured at single presynaptic boutons of cerebellar granule cell ascending axons and parallel fibers
- PMID: 20049574
- PMCID: PMC3411191
- DOI: 10.1007/s12311-009-0151-3
Calcium influx measured at single presynaptic boutons of cerebellar granule cell ascending axons and parallel fibers
Abstract
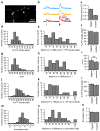
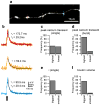
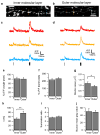
Action potential-evoked calcium influx into presynaptic boutons is a key determinant of synaptic strength and function. Here, we have examined the calcium dynamics at individual presynaptic boutons of the cerebellar granule cells in the molecular layer of cerebellar slices and investigated whether different subpopulations of granule cell boutons exhibit different calcium dynamics. We found that a population of boutons with low basal calcium clearance rates may activate a second clearance mechanism and exhibit biphasic calcium decay on high calcium influx induced by bursts of action potentials. We also found that boutons on ascending axons and parallel fibers show similar calcium influx amplitudes and calcium clearance rates in response to action potentials. Lastly, we found that parallel fiber boutons located in the inner molecular layer have a higher calcium clearance rate than boutons located in the outer molecular layer. These results suggest that cerebellar granule cell boutons should not be regarded as a homogeneous population, but rather that different subpopulations of boutons may exhibit different properties. The heterogeneity of presynaptic boutons may allow different learned behaviors to be encoded in the same circuit without mutual interference and may be a general mechanism for increasing the computational capacity of the brain.
Figures

References
-
- Napper RM, Harvey RJ. Number of parallel fiber synapses on an individual Purkinje cell in the cerebellum of the rat. J Comp Neurol. 1988;274(2):168–177. - PubMed
-
- Palay SL, Chan-Palay V. Cerebellar cortex: cytology and organization. Springer-Verlag, Berlin; 1974.
-
- Braitenberg V, Atwood RP. Morphological observations on the cerebellar cortex. J Comp Neurol. 1958;109(1):1–33. - PubMed
-
- Eccles JC, Ito M, Szentagothai J. The cerebellum as a neuronal machine. Springer; Berlin: 1967.
-
- Eccles JC, Llinas R, Sasaki K. Parallel fibre stimulation and the responses induced thereby in the Purkinje cells of the cerebellum. Exp Brain Res. 1966;1(1):17–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

