Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice
- PMID: 20049711
- PMCID: PMC3378118
- DOI: 10.1002/emmm.200900009
Neutralization of IFNgamma defeats haemophagocytosis in LCMV-infected perforin- and Rab27a-deficient mice
Abstract
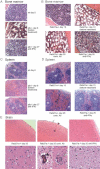
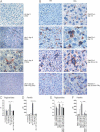
Hereditary haemophagocytic lymphohistiocytosis (HLH) is a fatal inflammatory disease and treatments currently may lead to serious side effects. There is a pressing need for effective, less toxic treatments for this disease. Previous reports have suggested that interferon gamma (IFNgamma) has a role in the pathogenesis of HLH. Here, we report that blocking IFNgamma had a therapeutic effect in two different murine models of human hereditary HLH (perforin-deficient and Rab27a-deficient mice, both infected with lymphocytic choriomeningitis virus). Therapeutic administration of an anti-IFNgamma antibody induced recovery from haemophagocytosis in both genetic models, as evidenced by increased survival in perforin-deficient mice and correction of blood cytopenia, moderation of body temperature changes, decreased cytokinaemia, restoration of splenic architecture and reduced haemophagocytosis in the liver of both murine models. Involvement of the central nervous system in Rab27a-deficient mice was prevented by anti-IFNgamma therapy. Hepatic T-cell infiltrates and virus persisted, with no detectable harm during the time course of these studies. These data strongly suggest that neutralization of IFNgamma could be used in humans to safely alleviate the clinical manifestations of haemophagocytosis.
Figures



References
-
- Abbott NJ, Ronnback L, Hansson E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. - PubMed
-
- Badovinac VP, Hamilton SE, Harty JT. Viral infection results in massive CD8+ T cell expansion and mortality in vaccinated perforin-deficient mice. Immunity. 2003;18:463–474. - PubMed
-
- Battegay M, Cooper S, Althage A, Banziger J, Hengartner H, Zinkernagel RM. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J Virol Methods. 1991;33:191–198. - PubMed
-
- Billiau AD, Roskams T, Van Damme-Lombaerts R, Matthys P, Wouters C. Macrophage activation syndrome: characteristic findings on liver biopsy illustrating the key role of activated, IFN-gamma-producing lymphocytes and IL-6- and TNF-alpha-producing macrophages. Blood. 2005;105:1648–1651. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

