Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion
- PMID: 20049737
- PMCID: PMC3378144
- DOI: 10.1002/emmm.200900039
Human colon cancer epithelial cells harbour active HEDGEHOG-GLI signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion
Abstract
Human colon cancers often start as benign adenomas through loss of APC, leading to enhanced beta CATENIN (beta CAT)/TCF function. These early lesions are efficiently managed but often progress to invasive carcinomas and incurable metastases through additional changes, the nature of which is unclear. We find that epithelial cells of human colon carcinomas (CCs) and their stem cells of all stages harbour an active HH-GLI pathway. Unexpectedly, they acquire a high HEDGEHOG-GLI (HH-GLI) signature coincident with the development of metastases. We show that the growth of CC xenografts, their recurrence and metastases require HH-GLI function, which induces a robust epithelial-to-mesenchymal transition (EMT). Moreover, using a novel tumour cell competition assay we show that the self-renewal of CC stem cells in vivo relies on HH-GLI activity. Our results indicate a key and essential role of the HH-GLI1 pathway in promoting CC growth, stem cell self-renewal and metastatic behavior in advanced cancers. Targeting HH-GLI1, directly or indirectly, is thus predicted to decrease tumour bulk and eradicate CC stem cells and metastases.
Figures
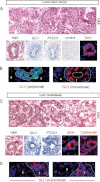
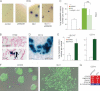
Analysis of CCs localized in the colon. Hematoxylin and eosin (H&E) staining, in situ hybridization for /GLI1/ and /PTCH1/ mRNAs (blue; CC7) and SHH (red; CC14) in (A) and GLI1 protein (green; CC6) in (B) in CC epithelial cells as indicated.
Analysis of metastatic CCs localized in the liver. H&E staining, in situ hybridization for /GLI1/ and /PTCH1/ mRNAs (blue; mCC2), SHH (red; mCC11) and cytokeratin (red; mCC11) in (C) and GLI1 protein (green; mCC1) in (D) in CC epithelial cells as indicated.
The top panels of (A, C) show wider views of the local CC6 and metastatic mCC2 tumors, depicting local colon invasion and high-grade dysplastic morphology (A) and liver invasion (C). Nuclei (blue) are stained with DAPI immunofluorescence images for SHH, cytokeratin and GLI1. l: liver cells, s: stroma; t: tumor. Scale bar = 50 µm for all panels.

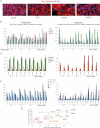
Heat map of gene expression determined by RT-qPCR shown as CD133+/CD133− expression ratios. CC are TNM staged. Numerical values are given in Fig S2 of Supporting Information. Here and in all figures expression levels were normalized with the geometric mean of the ct values of EEFIa and GAPDH. Ls = LS174T, HT = HT29, m = metastatic tumour in the liver.
Histograms of individual gene expression changes in CD133+ (red bars) and CD133− (blue bars) cells. The graphs use the same samples as in (A). x = mCC17 xenograft. Numerals refer to TNM stages. nc: normal colon; nl: normal liver; m = metastases.
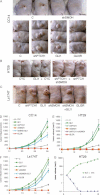
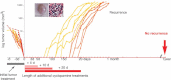
Comment in
-
Hedgehog signalling in colon cancer and stem cells.EMBO Mol Med. 2009 Sep;1(6-7):300-2. doi: 10.1002/emmm.200900042. EMBO Mol Med. 2009. PMID: 20049733 Free PMC article.
-
Mouse models and mouse supermodels.EMBO Mol Med. 2010 Oct;2(10):385-6; author reply 386-7. doi: 10.1002/emmm.201000090. EMBO Mol Med. 2010. PMID: 20721989 Free PMC article. No abstract available.
Similar articles
-
Hedgehog signaling and the Gli code in stem cells, cancer, and metastases.Sci Signal. 2011 Nov 22;4(200):pt9. doi: 10.1126/scisignal.2002540. Sci Signal. 2011. PMID: 22114144
-
Hedgehog signalling in colon cancer and stem cells.EMBO Mol Med. 2009 Sep;1(6-7):300-2. doi: 10.1002/emmm.200900042. EMBO Mol Med. 2009. PMID: 20049733 Free PMC article.
-
Loss of WNT-TCF addiction and enhancement of HH-GLI1 signalling define the metastatic transition of human colon carcinomas.EMBO Mol Med. 2010 Nov;2(11):440-57. doi: 10.1002/emmm.201000098. EMBO Mol Med. 2010. PMID: 20941789 Free PMC article.
-
Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals.J Mol Cell Biol. 2010 Apr;2(2):84-95. doi: 10.1093/jmcb/mjp052. Epub 2010 Jan 17. J Mol Cell Biol. 2010. PMID: 20083481 Free PMC article. Review.
-
Cooperative integration between HEDGEHOG-GLI signalling and other oncogenic pathways: implications for cancer therapy.Expert Rev Mol Med. 2015 Feb 9;17:e5. doi: 10.1017/erm.2015.3. Expert Rev Mol Med. 2015. PMID: 25660620 Free PMC article. Review.
Cited by
-
The utility of hedgehog signaling pathway inhibition for cancer.Oncologist. 2012;17(8):1090-9. doi: 10.1634/theoncologist.2011-0450. Epub 2012 Jul 31. Oncologist. 2012. PMID: 22851551 Free PMC article. Review.
-
Anti-Cancer Stem-like Cell Compounds in Clinical Development - An Overview and Critical Appraisal.Front Oncol. 2016 May 10;6:115. doi: 10.3389/fonc.2016.00115. eCollection 2016. Front Oncol. 2016. PMID: 27242955 Free PMC article. Review.
-
Loss of TCR-beta F1 and/or EZRIN expression is associated with unfavorable prognosis in nodal peripheral T-cell lymphomas.Blood Cancer J. 2013 Apr 19;3(4):e111. doi: 10.1038/bcj.2013.10. Blood Cancer J. 2013. PMID: 23599023 Free PMC article.
-
GLI3 knockdown decreases stemness, cell proliferation and invasion in oral squamous cell carcinoma.Int J Oncol. 2018 Dec;53(6):2458-2472. doi: 10.3892/ijo.2018.4572. Epub 2018 Sep 26. Int J Oncol. 2018. PMID: 30272273 Free PMC article.
-
The Role of Sonic Hedgehog Signaling in the Tumor Microenvironment of Oral Squamous Cell Carcinoma.Int J Mol Sci. 2019 Nov 17;20(22):5779. doi: 10.3390/ijms20225779. Int J Mol Sci. 2019. PMID: 31744214 Free PMC article.
References
-
- Alinger B, Kiesslich T, Datz C, Aberger F, Strasser F, Berr F, Dietze O, Kaserer K, Hauser-Kronberger C. Hedgehog signaling is involved in differentiation of normal colonic tissue rather than in tumor proliferation. Virchows Arch. 2009;454:369–379. - PubMed
-
- Batlle E, Sancho E, Francí C, Domínguez D, Monfar M, Baulida J, García De Herreros A. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
-
- Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, Parker AR, Shimada Y, Eshleman JR, Watkins DN, et al. Widespread requirement for Hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

