Nanoparticle-based biologic mimetics
- PMID: 20049778
- PMCID: PMC2839134
- DOI: 10.1002/wnan.20
Nanoparticle-based biologic mimetics
Abstract
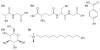
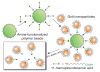
Centered on solid chemistry foundations, biology and materials science have reached a crossroad where bottom-up designs of new biologically important nanomaterials are a reality. The topics discussed here present the interdisciplinary field of creating biological mimics. Specifically, this discussion focuses on mimics that are developed using various types of metal nanoparticles (particularly gold) through facile synthetic methods. These methods conjugate biologically relevant molecules, e.g., small molecules, peptides, proteins, and carbohydrates, in conformationally favorable orientations on the particle surface. These new products provide stable, safe, and effective substitutes for working with potentially hazardous biologicals for applications such as drug targeting, immunological studies, biosensor development, and biocatalysis. Many standard bioanalytical techniques can be used to characterize and validate the efficacy of these new materials, including quartz crystal microbalance (QCM), surface plasmon resonance (SPR), and enzyme-linked immunosorbent assay (ELISA). Metal nanoparticle-based biomimetics continue to be developed as potential replacements for the native biomolecule in applications of immunoassays and catalysis.
Figures





Similar articles
-
Advance and prospect of bionanomaterials.Biotechnol Prog. 2003 May-Jun;19(3):683-92. doi: 10.1021/bp025791i. Biotechnol Prog. 2003. Retraction in: Biotechnol Prog. 2005 Mar-Apr;21(2):650. doi: 10.1021/bp0580804. PMID: 12790626 Retracted. Review.
-
Quartz crystal microbalance based nanosensor for lysozyme detection with lysozyme imprinted nanoparticles.Biosens Bioelectron. 2010 Oct 15;26(2):815-21. doi: 10.1016/j.bios.2010.06.003. Epub 2010 Jun 11. Biosens Bioelectron. 2010. PMID: 20605089
-
Ultrasensitive detection of testosterone using conjugate linker technology in a nanoparticle-enhanced surface plasmon resonance biosensor.Biosens Bioelectron. 2009 Mar 15;24(7):2177-83. doi: 10.1016/j.bios.2008.11.018. Epub 2008 Nov 30. Biosens Bioelectron. 2009. PMID: 19117747
-
Antigen-antibody interaction from quartz crystal microbalance immunosensors based on magnetic CoFe2O4/SiO2 composite nanoparticle-functionalized biomimetic interface.Bioprocess Biosyst Eng. 2007 Jul;30(4):243-9. doi: 10.1007/s00449-007-0120-5. Bioprocess Biosyst Eng. 2007. PMID: 17354012
-
Nanotechnology and biomimetics with 2-D protein crystals.IEEE Eng Med Biol Mag. 2003 May-Jun;22(3):140-50. doi: 10.1109/memb.2003.1213637. IEEE Eng Med Biol Mag. 2003. PMID: 12845830 Review. No abstract available.
Cited by
-
Characterization of thiolate-protected gold nanoparticles by mass spectrometry.Analyst. 2010 May;135(5):868-74. doi: 10.1039/b922291j. Epub 2010 Feb 9. Analyst. 2010. PMID: 20419232 Free PMC article. Review.
-
Exploration of possible binding sites of nanoparticles on protein by cross-linking chemistry coupled with mass spectrometry.Anal Chem. 2011 Sep 15;83(18):6929-34. doi: 10.1021/ac201889j. Epub 2011 Aug 29. Anal Chem. 2011. PMID: 21870789 Free PMC article.
-
Effects of Noncovalent Interactions on the Catalytic Activity of Unsupported Colloidal Palladium Nanoparticles Stabilized with Thiolate Ligands.J Phys Chem C Nanomater Interfaces. 2017 Sep 28;121(38):20882-20891. doi: 10.1021/acs.jpcc.7b07109. Epub 2017 Sep 6. J Phys Chem C Nanomater Interfaces. 2017. PMID: 29326755 Free PMC article.
-
Icam-1 targeted nanogels loaded with dexamethasone alleviate pulmonary inflammation.PLoS One. 2014 Jul 14;9(7):e102329. doi: 10.1371/journal.pone.0102329. eCollection 2014. PLoS One. 2014. PMID: 25019304 Free PMC article.
-
Triamcinolone acetonide-loaded nanoparticles encapsulated by CD90+ MCSs-derived microvesicles drive anti-inflammatory properties and promote cartilage regeneration after osteoarthritis.J Nanobiotechnology. 2022 Mar 19;20(1):150. doi: 10.1186/s12951-022-01367-z. J Nanobiotechnology. 2022. PMID: 35305656 Free PMC article.
References
-
- Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatized gold nanoparticles in a two-phase liquid-liquid system. Chem Commun. 1994;7:801–902.
-
- Hostetler MJ, Green SJ, Stokes JJ, Murray RW. Monolayers in three dimensions: synthesis and electrochemistry of omega-functionalized alkanethiolate-stabilized gold cluster compounds. J Am Chem Soc. 1996;118(17):4212–4213.
-
- Templeton AC, Hostetler MJ, Warmoth EK, Chen S, Hartshorn CM, et al. Gateway reactions to diverse, polyfunctional monolayer-protected gold clusters. J Am Chem Soc. 1998;120:4845–4849.
-
- Faraday M. Experimental relations of gold (and other metals) to light. Philos Trans R Soc Lond. 1857;147:145–181.
-
- Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

