Mechanism of formation of the C-terminal beta-hairpin of the B3 domain of the immunoglobulin-binding protein G from Streptococcus. IV. Implication for the mechanism of folding of the parent protein
- PMID: 20049918
- PMCID: PMC2829375
- DOI: 10.1002/bip.21365
Mechanism of formation of the C-terminal beta-hairpin of the B3 domain of the immunoglobulin-binding protein G from Streptococcus. IV. Implication for the mechanism of folding of the parent protein
Abstract
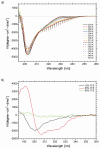
A 34-residue alpha/beta peptide [IG(28-61)], derived from the C-terminal part of the B3 domain of the immunoglobulin binding protein G from Streptoccocus, was studied using CD and NMR spectroscopy at various temperatures and by differential scanning calorimetry. It was found that the C-terminal part (a 16-residue-long fragment) of this peptide, which corresponds to the sequence of the beta-hairpin in the native structure, forms structure similar to the beta-hairpin only at T = 313 K, and the structure is stabilized by non-native long-range hydrophobic interactions (Val47-Val59). On the other hand, the N-terminal part of IG(28-61), which corresponds to the middle alpha-helix in the native structure, is unstructured at low temperature (283 K) and forms an alpha-helix-like structure at 305 K, and only one helical turn is observed at 313 K. At all temperatures at which NMR experiments were performed (283, 305, and 313 K), we do not observe any long-range connectivities which would have supported packing between the C-terminal (beta-hairpin) and the N-terminal (alpha-helix) parts of the sequence. Such interactions are absent, in contrast to the folding pathway of the B domain of protein G, proposed recently by Kmiecik and Kolinski (Biophys J 2008, 94, 726-736), based on Monte-Carlo dynamics studies. Alternative folding mechanisms are proposed and discussed.
Figures



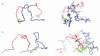
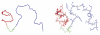

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

