Delivery of therapeutic proteins
- PMID: 20049941
- PMCID: PMC2857543
- DOI: 10.1002/jps.22054
Delivery of therapeutic proteins
Abstract
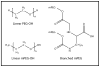
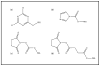
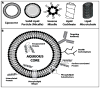
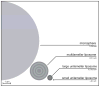
The safety and efficacy of protein therapeutics are limited by three interrelated pharmaceutical issues, in vitro and in vivo instability, immunogenicity and shorter half-lives. Novel drug modifications for overcoming these issues are under investigation and include covalent attachment of poly(ethylene glycol) (PEG), polysialic acid, or glycolic acid, as well as developing new formulations containing nanoparticulate or colloidal systems (e.g., liposomes, polymeric microspheres, polymeric nanoparticles). Such strategies have the potential to develop as next generation protein therapeutics. This review includes a general discussion on these delivery approaches.
(c) 2010 Wiley-Liss, Inc. and the American Pharmacists Association
Figures
References
-
- Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–2204. - PubMed
-
- Malik NN. Drug discovery: past, present and future. Drug Discov Today. 2008;13(21–22):909–912. - PubMed
-
- Almeida AJ, Souto E. Solid lipid nanoparticles as a drug delivery system for peptides and proteins. Adv Drug Deliv Rev. 2007;59(6):478–490. - PubMed
-
- Banakar UV. Advances and opportunities in delivery of therapeutic proteins and peptides. J Biomater Appl. 1997;11 (4):377–429. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

