Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double-blind, randomized study
- PMID: 20049950
- PMCID: PMC2972638
- DOI: 10.1002/ibd.21193
Direct comparison of two different mesalamine formulations for the induction of remission in patients with ulcerative colitis: a double-blind, randomized study
Abstract
Background: Mesalamine is the first-line drug for the treatment of ulcerative colitis (UC). We directly compared the efficacy and safety of two mesalamine formulations for the induction of remission in patients with UC.
Methods: In a multicenter, double-blind, randomized study, 229 patients with mild-to-moderate active UC were assigned to 4 groups: 66 and 65 received a pH-dependent release formulation of 2.4 g/day (pH-2.4 g) or 3.6 g/day (pH-3.6 g), respectively; 65 received a time-dependent release formulation of 2.25 g/day (Time-2.25 g), and 33 received placebo (Placebo). The drugs were administered three times daily for eight weeks. The primary endpoint was a decrease in the UC disease activity index (UC-DAI).
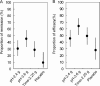
Results: In the full analysis set (n = 225) the decrease in UC-DAI in each group was 1.5 in pH-2.4 g, 2.9 in pH-3.6 g, 1.3 in Time-2.25 g and 0.3 in Placebo, respectively. These results demonstrate the superiority of pH-3.6 g over Time-2.25 g (P = 0.003) and the noninferiority of pH-2.4 g to Time-2.25 g. Among the patients with proctitis-type UC, a significant decrease in UC-DAI was observed in pH-2.4 g and pH-3.6 g as compared to Placebo, but not in Time-2.25 g. No differences were observed in the safety profiles.
Conclusions: Higher dose of the pH-dependent release formulation was more effective for induction of remission in patients with mild-to-moderate active UC. Additionally, the pH-dependent release formulation was preferable to the time-dependent release formulation for patients with proctitis-type UC (UMIN Clinical Trials Registry, no. C000000288).
Figures

Similar articles
-
Direct comparison of two different mesalamine formulations for the maintenance of remission in patients with ulcerative colitis: a double-blind, randomized study.Inflamm Bowel Dis. 2010 Sep;16(9):1575-82. doi: 10.1002/ibd.21194. Inflamm Bowel Dis. 2010. PMID: 20049949 Free PMC article. Clinical Trial.
-
Once-daily budesonide MMX® extended-release tablets induce remission in patients with mild to moderate ulcerative colitis: results from the CORE I study.Gastroenterology. 2012 Nov;143(5):1218-1226.e2. doi: 10.1053/j.gastro.2012.08.003. Epub 2012 Aug 11. Gastroenterology. 2012. PMID: 22892337 Clinical Trial.
-
Comparison of QD and TID oral mesalazine for maintenance of remission in quiescent ulcerative colitis: a double-blind, double-dummy, randomized multicenter study.Inflamm Bowel Dis. 2013 Jul;19(8):1681-90. doi: 10.1097/MIB.0b013e318286fa3d. Inflamm Bowel Dis. 2013. PMID: 23624890 Clinical Trial.
-
Mesalamine with MMX technology for the treatment of ulcerative colitis.Expert Rev Gastroenterol Hepatol. 2008 Jun;2(3):299-314. doi: 10.1586/17474124.2.3.299. Expert Rev Gastroenterol Hepatol. 2008. PMID: 19072380 Review.
-
Mesalamine in the treatment and maintenance of remission of ulcerative colitis.Expert Rev Clin Pharmacol. 2012 Mar;5(2):113-23. doi: 10.1586/ecp.12.2. Expert Rev Clin Pharmacol. 2012. PMID: 22390554 Free PMC article. Review.
Cited by
-
Efficacy of oral prolonged-release mesalazine in moderately active ulcerative colitis.JGH Open. 2023 Jul 7;7(7):516-519. doi: 10.1002/jgh3.12935. eCollection 2023 Jul. JGH Open. 2023. PMID: 37496812 Free PMC article.
-
Efficacy of a pH-dependent controlled-release mesalazine based on clinical and endoscopic assessment for ulcerative colitis: a retrospective cohort study.Clin Exp Gastroenterol. 2015 Jul 31;8:225-30. doi: 10.2147/CEG.S86528. eCollection 2015. Clin Exp Gastroenterol. 2015. PMID: 26347309 Free PMC article.
-
Long-term efficacy and safety of once-daily mesalazine granules for the treatment of active ulcerative colitis.Clin Exp Gastroenterol. 2014 Sep 23;7:369-83. doi: 10.2147/CEG.S35691. eCollection 2014. Clin Exp Gastroenterol. 2014. PMID: 25285021 Free PMC article. Review.
-
Efficacy and safety of two pH-dependent-release mesalamine doses in moderately active ulcerative colitis: a multicenter, randomized, double-blind, parallel-group study.Intest Res. 2016 Jan;14(1):50-9. doi: 10.5217/ir.2016.14.1.50. Epub 2016 Jan 26. Intest Res. 2016. PMID: 26884735 Free PMC article.
-
Mesalazine preparations for the treatment of ulcerative colitis: Are all created equal?World J Gastrointest Pharmacol Ther. 2015 Nov 6;6(4):137-44. doi: 10.4292/wjgpt.v6.i4.137. World J Gastrointest Pharmacol Ther. 2015. PMID: 26558148 Free PMC article. Review.
References
-
- Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults (update): American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2004;99:1371–1385. - PubMed
-
- Brogden RN, Sorkin EM. Mesalazine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in chronic inflammatory bowel disease. Drugs. 1989;38:500–523. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
