Inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation
- PMID: 20050331
- PMCID: PMC2807489
- DOI: 10.1111/j.1365-2567.2009.03152.x
Inhibition of cyclooxygenase-2 impairs the expression of essential plasma cell transcription factors and human B-lymphocyte differentiation
Abstract
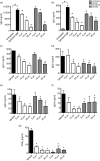
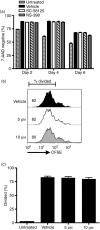
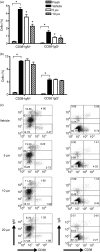
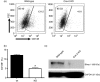
Cyclooxygenase (Cox) inhibitors are among the most widely used and commonly prescribed medications. Relatively little is understood about their influence on human immune responses. Herein, we discovered a novel and important mechanism whereby non-steroidal anti-inflammatory drugs (NSAIDs) blunt human B-cell antibody production. We demonstrate that the Cox-2 selective small molecule inhibitors SC-58125 and NS-398 attenuate the production of human antibody isotypes including immunoglobulin M (IgM), IgG1, IgG2, IgG3 and IgG4. In addition, inhibition of Cox-2 significantly reduced the generation of CD38+ IgM+ and CD38+ IgG+ antibody-secreting cells. Interestingly, we discovered that inhibition of Cox-2 activity in normal human B cells severely reduced the messenger RNA and protein levels of the essential plasma cell transcription factor, Blimp-1. These observations were mirrored in Cox-2-deficient mice, which had reduced CD138+ plasma cells and a near loss of Blimp-1 expression. These new findings demonstrate a critical role for Cox-2 in the terminal differentiation of human B lymphocytes to antibody-secreting plasma cells. The use of NSAIDs may adversely influence the efficacy of vaccines, especially in the immunocompromised, elderly and when vaccines are weakly immunogenic.
Figures

Similar articles
-
Ibuprofen and other widely used non-steroidal anti-inflammatory drugs inhibit antibody production in human cells.Cell Immunol. 2009;258(1):18-28. doi: 10.1016/j.cellimm.2009.03.007. Epub 2009 Apr 5. Cell Immunol. 2009. PMID: 19345936 Free PMC article.
-
Activated human B lymphocytes express cyclooxygenase-2 and cyclooxygenase inhibitors attenuate antibody production.J Immunol. 2005 Mar 1;174(5):2619-26. doi: 10.4049/jimmunol.174.5.2619. J Immunol. 2005. PMID: 15728468
-
Cyclooxygenase-2 independent effects of cyclooxygenase-2 inhibitors on oxidative stress and intracellular glutathione content in normal and malignant human B-cells.Cancer Immunol Immunother. 2008 Mar;57(3):347-58. doi: 10.1007/s00262-007-0374-4. Epub 2007 Aug 1. Cancer Immunol Immunother. 2008. PMID: 17668203 Free PMC article.
-
Dual acting anti-inflammatory drugs: a reappraisal.Pharmacol Res. 2001 Dec;44(6):437-50. doi: 10.1006/phrs.2001.0872. Pharmacol Res. 2001. PMID: 11735348 Review.
-
New NSAIDs and gastroduodenal damage.Ital J Gastroenterol. 1996 Dec;28 Suppl 4:28-9. Ital J Gastroenterol. 1996. PMID: 9032579 Review.
Cited by
-
Non-steroidal anti-inflammatory drugs dampen the cytokine and antibody response to SARS-CoV-2 infection.J Virol. 2021 Mar 10;95(7):e00014-21. doi: 10.1128/JVI.00014-21. Epub 2021 Jan 13. J Virol. 2021. PMID: 33441348 Free PMC article.
-
Lipoxin B4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression.J Immunol. 2018 Dec 1;201(11):3343-3351. doi: 10.4049/jimmunol.1700503. Epub 2018 Oct 22. J Immunol. 2018. PMID: 30348736 Free PMC article.
-
D1-like dopamine receptors promote B-cell differentiation in systemic lupus erythematosus.Cell Commun Signal. 2024 Oct 17;22(1):502. doi: 10.1186/s12964-024-01885-3. Cell Commun Signal. 2024. PMID: 39420360 Free PMC article.
-
Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells.J Immunol. 2012 Jul 15;189(2):1036-42. doi: 10.4049/jimmunol.1103483. Epub 2012 Jun 18. J Immunol. 2012. PMID: 22711890 Free PMC article.
-
Inhibition of COX-2 ameliorates murine liver schistosomiasis japonica through splenic cellular immunoregulation.Parasit Vectors. 2022 Apr 23;15(1):144. doi: 10.1186/s13071-022-05201-1. Parasit Vectors. 2022. PMID: 35461268 Free PMC article.
References
-
- Martins G, Calame K. Regulation and functions of Blimp-1 in T and B lymphocytes. Annu Rev Immunol. 2008;26:133–69. - PubMed
-
- Shapiro-Shelef M, Calame K. Regulation of plasma-cell development. Nat Rev Immunol. 2005;5:230–42. - PubMed
-
- Shapiro-Shelef M, Lin KI, McHeyzer-Williams LJ, Liao J, McHeyzer-Williams MG, Calame K. Blimp-1 is required for the formation of immunoglobulin secreting plasma cells and pre-plasma memory B cells. Immunity. 2003;19:607–20. - PubMed
-
- Lindroth K, Fernandez C. The role of Blimp-1 in the GC reaction: differential expression of Blimp-1 upon immunization with TD and TI antigens. Immunol Lett. 2007;113:70–5. - PubMed
-
- Soro PG, Morales AP, Martinez MJ, Morales AS, Copin SG, Marcos MA, Gaspar ML. Differential involvement of the transcription factor Blimp-1 in T cell-independent and -dependent B cell differentiation to plasma cells. J Immunol. 1999;163:611–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

