Physiological activity of neuropeptide f on the hindgut of the blood-feeding hemipteran, Rhodnius prolixus
- PMID: 20050776
- PMCID: PMC3011914
- DOI: 10.1673/031.009.5701
Physiological activity of neuropeptide f on the hindgut of the blood-feeding hemipteran, Rhodnius prolixus
Abstract
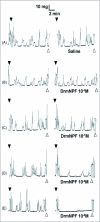
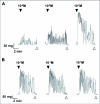
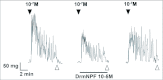
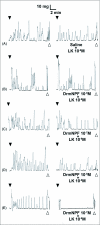
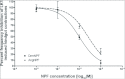
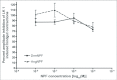
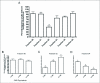
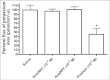
Current hypotheses propose that, in the invertebrates, neuropeptide F (NPF), the vertebrate NPY homologue, may be capable of regulating responses to diverse cues related to nutritional status and feeding. An investigation into the effects of Drosophila melanogaster NPF (DrmNPF) and Anopheles gambiae NPF (AngNPF) on hindgut physiology of Rhodnius prolixus Stal (Heimptera: Reduviidae) suggests a myoinhibitory role for these peptides and the R. prolixus native peptide. Extracts of the central nervous system of R. prolixus were processed and several HPLC-fractions revealed NPF-like activity within the nanomolar equivalent range when tested using the hindgut contraction assay. Although NPF has been shown to decrease epithelial membrane potential in Aedes aegypti larval midgut preparations, NPF does not appear to play a role in epithelial transport of potassium in the hindgut. While the function of NPF has yet to be established, NPF-like effects suggest multiple physiological roles for NPF among invertebrates.
Figures


References
-
- Brown MR, Raikhel AS, Lea AO. Ultrastructure of midgut endocrine cells in the adult mosquito, Aedes aegypti. Tissue and Cell. 1985;17:709–721. - PubMed
-
- Donini A, O'Donnell MJ. Analysis of Na+, Cl-, K+, H+ and NH4+ concentration gradients adjacent to the surface of anal papillae of the mosquito Aedes aegypti: application of self-referencing ion-selective microelectrodes. Journal of Experimental Biology. 2005;208:603–610. - PubMed
-
- Gonzalez R, Orchard I. Characterization of neuropeptide F-like immunoreactivity in the blood-feeding hemipteran, Rhodnius prolixus. Peptides. 2008;29:545–558. - PubMed
-
- Halton DW, Shaw C, Maule AG, Smart D. Regulatory peptides in helminth parasites. Advances in Parasitology. 1994;34:163–227. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

