High efficacy and minimal peptide required for the anti-angiogenic and anti-hepatocarcinoma activities of plasminogen K5
- PMID: 20050964
- PMCID: PMC3823168
- DOI: 10.1111/j.1582-4934.2009.01004.x
High efficacy and minimal peptide required for the anti-angiogenic and anti-hepatocarcinoma activities of plasminogen K5
Abstract
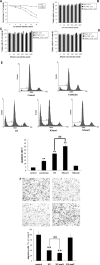
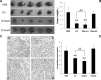
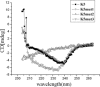
Kringle 5(K5) is the fifth kringle domain of human plasminogen and its anti-angiogenic activity is more potent than angiostatin that includes the first four kringle fragment of plasminogen. Our recent study demonstrated that K5 suppressed hepatocarcinoma growth by anti-angiogenesis. To find high efficacy and minimal peptide sequence required for the anti-angiogenic and anti-tumour activities of K5, two deletion mutants of K5 were generated. The amino acid residues outside kringle domain of intact K5 (Pro452-Ala542) were deleted to form K5mut1(Cys462-Cys541). The residue Cys462 was deleted again to form K5mut2(Met463-Cys541). K5mut1 specifically inhibited proliferation, migration and induced apoptosis of endothelial cells, with an apparent two-fold enhanced activity than K5. Intraperitoneal injection of K5mut1 resulted in more potent tumour growth inhibition and microvessel density reduction than K5 both in HepA-grafted and Bel7402-xenografted hepatocarcinoma mouse models. These results suggested that K5mut1 has more potent anti-angiogenic activity than intact K5. K5mut2, which lacks only the amino terminal cysteine of K5mut1, completely lost the activity, suggesting that the kringle domain is essential for the activity of K5. The activity was enhanced to K5mut1 level when five acidic amino acids of K5 in NH(2) terminal outside kringle domain were replaced by five serine residues (K5mut3). The shielding effect of acidic amino acids may explain why K5mut1 has higher activity. K5, K5mut1 and K5mut3 held characteristic β-sheet spectrum while K5mut2 adopted random coil structure. These results suggest that K5mut1 with high efficacy is the minimal active peptide sequence of K5 and may have therapeutic potential in liver cancer.
© 2009 The Authors Journal compilation © 2010 Foundation for Cellular and Molecular Medicine/Blackwell Publishing Ltd.
Figures




Similar articles
-
Dual inhibition of plasminogen kringle 5 on angiogenesis and chemotaxis suppresses tumor metastasis by targeting HIF-1α pathway.PLoS One. 2012;7(12):e53152. doi: 10.1371/journal.pone.0053152. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300882 Free PMC article.
-
Kringle 5 of human plasminogen suppresses hepatocellular carcinoma growth both in grafted and xenografted mice by anti-angiogenic activity.Cancer Biol Ther. 2006 Apr;5(4):399-405. doi: 10.4161/cbt.5.4.2511. Epub 2006 Apr 14. Cancer Biol Ther. 2006. PMID: 16481742
-
Selective inhibition by kringle 5 of human plasminogen on endothelial cell migration, an important process in angiogenesis.Biochem Biophys Res Commun. 1998 Jun 18;247(2):414-9. doi: 10.1006/bbrc.1998.8825. Biochem Biophys Res Commun. 1998. PMID: 9642142
-
Angiostatin and angiostatin-related proteins.Cancer Metastasis Rev. 2000;19(1-2):97-107. doi: 10.1023/a:1026525121027. Cancer Metastasis Rev. 2000. PMID: 11191071 Review.
-
Kringle structures and antiangiogenesis.Curr Med Chem Anticancer Agents. 2002 Nov;2(6):667-81. doi: 10.2174/1568011023353705. Curr Med Chem Anticancer Agents. 2002. PMID: 12678719 Review.
Cited by
-
Modification of cyclic NGR tumor neovasculature-homing motif sequence to human plasminogen kringle 5 improves inhibition of tumor growth.PLoS One. 2012;7(5):e37132. doi: 10.1371/journal.pone.0037132. Epub 2012 May 10. PLoS One. 2012. PMID: 22590653 Free PMC article.
-
Decreased K5 receptor expression in the retina, a potential pathogenic mechanism for diabetic retinopathy.Mol Vis. 2012;18:330-6. Epub 2012 Feb 4. Mol Vis. 2012. PMID: 22355244 Free PMC article.
-
TargetAntiAngio: A Sequence-Based Tool for the Prediction and Analysis of Anti-Angiogenic Peptides.Int J Mol Sci. 2019 Jun 17;20(12):2950. doi: 10.3390/ijms20122950. Int J Mol Sci. 2019. PMID: 31212918 Free PMC article.
-
Dual inhibition of plasminogen kringle 5 on angiogenesis and chemotaxis suppresses tumor metastasis by targeting HIF-1α pathway.PLoS One. 2012;7(12):e53152. doi: 10.1371/journal.pone.0053152. Epub 2012 Dec 31. PLoS One. 2012. PMID: 23300882 Free PMC article.
References
-
- Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. - PubMed
-
- Folkman J. Role of angiogenesis in tumor growth and metastasis. Semin Oncol. 2002;29:15–8. - PubMed
-
- Gao G, Li Y, Fant J, et al. Difference in ischemic regulation of vascular endothelial growth factor and pigment epithelium-derived factor in brown Norway and Sprague-Dawley rats contributing to different susceptibilities to retinal neovascularization. Diabetes. 2002;51:1218–25. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. - PubMed
-
- Cao Y. Antiangiogenic cancer therapy. Semin Cancer Biol. 2004;14:139–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

