Gangliosides and beta1-integrin are required for caveolae and membrane domains
- PMID: 20051050
- PMCID: PMC2852475
- DOI: 10.1111/j.1600-0854.2009.01022.x
Gangliosides and beta1-integrin are required for caveolae and membrane domains
Abstract
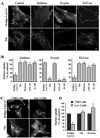
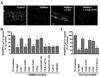
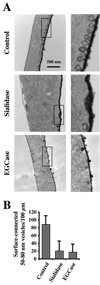
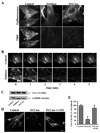
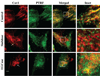
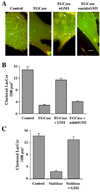
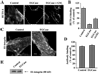
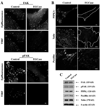
Caveolae are plasma membrane domains involved in the uptake of certain pathogens and toxins. Internalization of some cell surface integrins occurs via caveolae suggesting caveolae may play a crucial role in modulating integrin-mediated adhesion and cell migration. Here we demonstrate a critical role for gangliosides (sialo-glycosphingolipids) in regulating caveolar endocytosis in human skin fibroblasts. Pretreatment of cells with endoglycoceramidase (cleaves glycosphingolipids) or sialidase (modifies cell surface gangliosides and glycoproteins) selectively inhibited caveolar endocytosis by >70%, inhibited the formation of plasma membrane domains enriched in sphingolipids and cholesterol ('lipid rafts'), reduced caveolae and caveolin-1 at the plasma membrane by approximately 80%, and blunted activation of beta1-integrin, a protein required for caveolar endocytosis in these cells. These effects could be reversed by a brief incubation with gangliosides (but not with asialo-gangliosides or other sphingolipids) at 10 degrees C, suggesting that sialo-lipids are critical in supporting caveolar endocytosis. Endoglycoceramidase treatment also caused a redistribution of focal adhesion kinase, paxillin, talin, and PIP Kinase Igamma away from focal adhesions. The effects of sialidase or endoglycoceramidase on membrane domains and the distribution of caveolin-1 could be recapitulated by beta1-integrin knockdown. These results suggest that both gangliosides and beta1-integrin are required for maintenance of caveolae and plasma membrane domains.
Figures
Similar articles
-
Membrane microdomains, caveolae, and caveolar endocytosis of sphingolipids.Mol Membr Biol. 2006 Jan-Feb;23(1):101-10. doi: 10.1080/09687860500460041. Mol Membr Biol. 2006. PMID: 16611585 Review.
-
The glycosphingolipid, lactosylceramide, regulates beta1-integrin clustering and endocytosis.Cancer Res. 2005 Sep 15;65(18):8233-41. doi: 10.1158/0008-5472.CAN-05-0803. Cancer Res. 2005. PMID: 16166299
-
Inhibition of caveolar uptake, SV40 infection, and beta1-integrin signaling by a nonnatural glycosphingolipid stereoisomer.J Cell Biol. 2007 Mar 26;176(7):895-901. doi: 10.1083/jcb.200609149. Epub 2007 Mar 19. J Cell Biol. 2007. PMID: 17371832 Free PMC article.
-
Integrin-linked kinase controls microtubule dynamics required for plasma membrane targeting of caveolae.Dev Cell. 2010 Oct 19;19(4):574-88. doi: 10.1016/j.devcel.2010.09.007. Dev Cell. 2010. PMID: 20951348 Free PMC article.
-
Lipid rafts, caveolae, and their endocytosis.Int Rev Cell Mol Biol. 2010;282:135-63. doi: 10.1016/S1937-6448(10)82003-9. Epub 2010 Jun 18. Int Rev Cell Mol Biol. 2010. PMID: 20630468 Review.
Cited by
-
The epithelial calcium channel TRPV5 is regulated differentially by klotho and sialidase.J Biol Chem. 2013 Oct 11;288(41):29238-46. doi: 10.1074/jbc.M113.473520. Epub 2013 Aug 22. J Biol Chem. 2013. PMID: 23970553 Free PMC article.
-
Targeted Liposomes Encapsulating miR-603 Complexes Enhance Radiation Sensitivity of Patient-Derived Glioblastoma Stem-Like Cells.Pharmaceutics. 2021 Jul 21;13(8):1115. doi: 10.3390/pharmaceutics13081115. Pharmaceutics. 2021. PMID: 34452076 Free PMC article.
-
Reduced GM1 ganglioside in CFTR-deficient human airway cells results in decreased β1-integrin signaling and delayed wound repair.Am J Physiol Cell Physiol. 2014 May 1;306(9):C819-30. doi: 10.1152/ajpcell.00168.2013. Epub 2014 Feb 5. Am J Physiol Cell Physiol. 2014. PMID: 24500283 Free PMC article.
-
Lipid accumulation controls the balance between surface connection and scission of caveolae.Elife. 2020 May 4;9:e55038. doi: 10.7554/eLife.55038. Elife. 2020. PMID: 32364496 Free PMC article.
-
Systems-wide analysis unravels the new roles of CCM signal complex (CSC).Heliyon. 2019 Dec 2;5(12):e02899. doi: 10.1016/j.heliyon.2019.e02899. eCollection 2019 Dec. Heliyon. 2019. PMID: 31872111 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

