THOC5/FMIP, an mRNA export TREX complex protein, is essential for hematopoietic primitive cell survival in vivo
- PMID: 20051105
- PMCID: PMC2806247
- DOI: 10.1186/1741-7007-8-1
THOC5/FMIP, an mRNA export TREX complex protein, is essential for hematopoietic primitive cell survival in vivo
Abstract
Background: The transcription/export complex is evolutionarily conserved from yeast to man and is required for coupled transcription elongation and nuclear export of mRNAs. FMIP(Fms interacting protein) is a member of the THO (suppressors of the transcriptional defects of hpr1delta by overexpression) complex which is a subcomplex of the transcription/export complex. THO complex (THOC) components are not essential for bulk poly (A)+ RNA export in higher eukaryotes, but for the nuclear export of subset of mRNAs, however, their exact role is still unclear.
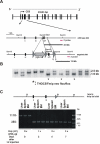
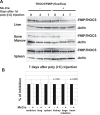
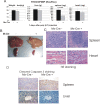
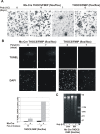
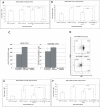
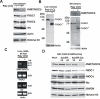
Results: To study the role of THOC5/Fms interacting protein in vivo, we generated THOC5/Fms interacting protein knockout mice. Since these mice are embryonic lethal, we then generated interferon inducible conditional THOC5/Fms interacting protein knockout mice. After three poly injections all of the mice died within 14 days. No pathological alterations, however, were observed in liver, kidney or heart. Thus we considered the hematopoietic system and found that seven days after poly injection, the number of blood cells in peripheral blood decreased drastically. Investigation of bone marrow cells showed that these became apoptotic within seven days after poly injection. Committed myeloid progenitor cells and cells with long term reconstituting potential were lost from bone marrow within four days after poly injection. Furthermore, infusion of normal bone marrow cells rescued mice from death induced by loss of THOC5/Fms interacting protein.
Conclusion: THOC5/Fms interacting protein is an essential element in the maintenance of hematopoiesis. Furthermore, mechanistically depletion of THOC5/Fms interacting protein causes the down-regulation of its direct interacting partner, THOC1 which may contribute to altered THO complex function and cell death.
Figures



Similar articles
-
THOC5, a member of the mRNA export complex, contributes to processing of a subset of wingless/integrated (Wnt) target mRNAs and integrity of the gut epithelial barrier.BMC Cell Biol. 2013 Nov 22;14:51. doi: 10.1186/1471-2121-14-51. BMC Cell Biol. 2013. PMID: 24267292 Free PMC article.
-
Identification of mRNAs that are spliced but not exported to the cytoplasm in the absence of THOC5 in mouse embryo fibroblasts.RNA. 2011 Jun;17(6):1048-56. doi: 10.1261/rna.2607011. Epub 2011 Apr 27. RNA. 2011. PMID: 21525145 Free PMC article.
-
Transcriptional regulation of immediate-early gene response by THOC5, a member of mRNA export complex, contributes to the M-CSF-induced macrophage differentiation.Cell Death Dis. 2013 Oct 24;4(10):e879. doi: 10.1038/cddis.2013.409. Cell Death Dis. 2013. PMID: 24157873 Free PMC article.
-
THOC5, a member of the mRNA export complex: a novel link between mRNA export machinery and signal transduction pathways in cell proliferation and differentiation.Cell Commun Signal. 2014 Jan 10;12:3. doi: 10.1186/1478-811X-12-3. Cell Commun Signal. 2014. PMID: 24410813 Free PMC article. Review.
-
mRNA export protein THOC5 as a tool for identification of target genes for cancer therapy.Cancer Lett. 2016 Apr 10;373(2):222-6. doi: 10.1016/j.canlet.2016.01.045. Epub 2016 Jan 28. Cancer Lett. 2016. PMID: 26828015 Review.
Cited by
-
Human TREX component Thoc5 affects alternative polyadenylation site choice by recruiting mammalian cleavage factor I.Nucleic Acids Res. 2013 Aug;41(14):7060-72. doi: 10.1093/nar/gkt414. Epub 2013 May 17. Nucleic Acids Res. 2013. PMID: 23685434 Free PMC article.
-
Differential expression of THOC1 and ALY mRNP biogenesis/export factors in human cancers.BMC Cancer. 2011 Feb 17;11:77. doi: 10.1186/1471-2407-11-77. BMC Cancer. 2011. PMID: 21329510 Free PMC article.
-
Lateral jaw motion in fish expands the functional repertoire of vertebrates and underpins the success of a dominant herbivore lineage.Proc Natl Acad Sci U S A. 2025 May 13;122(19):e2418982122. doi: 10.1073/pnas.2418982122. Epub 2025 May 5. Proc Natl Acad Sci U S A. 2025. PMID: 40324084
-
Intellectual disability associated with a homozygous missense mutation in THOC6.Orphanet J Rare Dis. 2013 Apr 26;8:62. doi: 10.1186/1750-1172-8-62. Orphanet J Rare Dis. 2013. PMID: 23621916 Free PMC article.
-
The THO Complex Coordinates Transcripts for Synapse Development and Dopamine Neuron Survival.Cell. 2018 Sep 6;174(6):1436-1449.e20. doi: 10.1016/j.cell.2018.07.046. Epub 2018 Aug 23. Cell. 2018. PMID: 30146163 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

