Non-invasive evaluation of nigrostriatal neuropathology in a proteasome inhibitor rodent model of Parkinson's disease
- PMID: 20051106
- PMCID: PMC2824797
- DOI: 10.1186/1471-2202-11-1
Non-invasive evaluation of nigrostriatal neuropathology in a proteasome inhibitor rodent model of Parkinson's disease
Abstract
Background: Predominantly, magnetic resonance imaging (MRI) studies in animal models of Parkinson's disease (PD) have focused on alterations in T2 water 1H relaxation or 1H MR spectroscopy (MRS), whilst potential morphological changes and their relationship to histological or behavioural outcomes have not been appropriately addressed. Therefore, in this study we have utilised MRI to scan in vivo brains from rodents bearing a nigrostriatal lesion induced by intranigral injection of the proteasome inhibitor lactacystin.
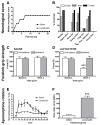
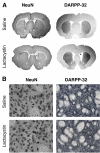
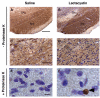
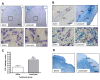
Results: Lactacystin induced parkinsonian-like behaviour, characterised by impaired contralateral forelimb grip strength and increased contralateral circling in response to apomorphine. T2-weighted MRI, 3-weeks post-lesion, revealed significant morphological changes in PD-relevant brain areas, including the striatum and ventral midbrain in addition to a decrease in T2 water 1H relaxation in the substantia nigra (SN), but not the striatum. Post-mortem histological analyses revealed extensive dopaminergic neuronal degeneration and alpha-synuclein aggregation in the SN. However, extensive neuronal loss could also be observed in extra-nigral areas, suggesting non-specific toxicity of lactacystin. Iron accumulation could also be observed throughout the midbrain reflecting changes in T2. Importantly, morphological, but not T2 relaxivity changes, were significantly associated with both behavioural and histological outcomes in this model.
Conclusions: A pattern of morphological changes in lactacystin-lesioned animals has been identified, as well as alterations in nigral T2 relaxivity. The significant relationship of morphological changes with behavioural and histological outcomes in this model raises the possibility that these may be useful non-invasive surrogate markers of nigrostriatal degeneration in vivo.
Figures




Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Evolution of extra-nigral damage predicts behavioural deficits in a rat proteasome inhibitor model of Parkinson's disease.PLoS One. 2011 Feb 25;6(2):e17269. doi: 10.1371/journal.pone.0017269. PLoS One. 2011. PMID: 21364887 Free PMC article.
-
Decreased behavioral response to intranigrally administered GABAA agonist muscimol in the lactacystin model of Parkinson's disease may result from partial lesion of nigral non-dopamine neurons: comparison to the classical neurotoxin 6-OHDA.Behav Brain Res. 2015 Apr 15;283:203-14. doi: 10.1016/j.bbr.2015.01.043. Epub 2015 Feb 2. Behav Brain Res. 2015. PMID: 25655509
-
Altered melatonin MT1 receptor expression in the ventral midbrain following 6-hydroxydopamine lesions in the rat medial forebrain bundle.Brain Res. 2016 Dec 1;1652:89-96. doi: 10.1016/j.brainres.2016.09.036. Epub 2016 Sep 28. Brain Res. 2016. PMID: 27693415
-
Morphological and metabolic changes in the nigro-striatal pathway of synthetic proteasome inhibitor (PSI)-treated rats: a MRI and MRS study.PLoS One. 2013;8(2):e56501. doi: 10.1371/journal.pone.0056501. Epub 2013 Feb 19. PLoS One. 2013. PMID: 23431380 Free PMC article.
Cited by
-
Physical therapy exerts sub-additive and suppressive effects on intracerebral neural stem cell implantation in a rat model of stroke.J Cereb Blood Flow Metab. 2022 May;42(5):826-843. doi: 10.1177/0271678X211062955. Epub 2021 Nov 26. J Cereb Blood Flow Metab. 2022. PMID: 34826373 Free PMC article.
-
Temporal-Spatial Profiling of Pedunculopontine Galanin-Cholinergic Neurons in the Lactacystin Rat Model of Parkinson's Disease.Neurotox Res. 2018 Jul;34(1):16-31. doi: 10.1007/s12640-017-9846-2. Epub 2017 Dec 7. Neurotox Res. 2018. PMID: 29218504
-
Nigral proteasome inhibition in mice leads to motor and non-motor deficits and increased expression of Ser129 phosphorylated α-synuclein.Front Behav Neurosci. 2015 Mar 31;9:68. doi: 10.3389/fnbeh.2015.00068. eCollection 2015. Front Behav Neurosci. 2015. PMID: 25873870 Free PMC article.
-
Parkinson's disease: experimental models and reality.Acta Neuropathol. 2018 Jan;135(1):13-32. doi: 10.1007/s00401-017-1788-5. Epub 2017 Nov 18. Acta Neuropathol. 2018. PMID: 29151169 Free PMC article. Review.
-
The Protective Effect of Repeated 1MeTIQ Administration on the Lactacystin-Induced Impairment of Dopamine Release and Decline in TH Level in the Rat Brain.Neurotox Res. 2018 Oct;34(3):706-716. doi: 10.1007/s12640-018-9939-6. Epub 2018 Aug 20. Neurotox Res. 2018. PMID: 30129004 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

