Schistosoma mansoni infection reduces the incidence of murine cerebral malaria
- PMID: 20051114
- PMCID: PMC2822789
- DOI: 10.1186/1475-2875-9-5
Schistosoma mansoni infection reduces the incidence of murine cerebral malaria
Abstract
Background: Plasmodium and Schistosoma are two of the most common parasites in tropical areas. Deregulation of the immune response to Plasmodium falciparum, characterized by a Th1 response, leads to cerebral malaria (CM), while a Th2 response accompanies chronic schistosomiasis.
Methods: The development of CM was examined in mice with concomitant Schistosoma mansoni and Plasmodium berghei ANKA infections. The effect of S. mansoni egg antigen injection on disease development and survival was also determined. Cytokine serum levels were estimated using ELISA. Statistical analysis was performed using t-test.
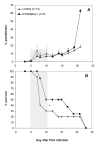
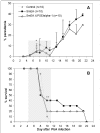
Results: The results demonstrate that concomitant S. mansoni and P. berghei ANKA infection leads to a reduction in CM. This effect is dependent on infection schedule and infecting cercariae number, and is correlated with a Th2 response. Schistosomal egg antigen injection delays the death of Plasmodium-infected mice, indicating immune involvement.
Conclusions: This research supports previous claims of a protective effect of helminth infection on CM development. The presence of multiple parasitic infections in patients from endemic areas should therefore be carefully noted in clinical trials, and in the development of standard treatment protocols for malaria. Defined helminth antigens may be considered for alleviation of immunopathological symptoms.
Figures
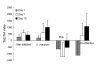
Similar articles
-
Schistosoma co-infection protects against brain pathology but does not prevent severe disease and death in a murine model of cerebral malaria.Int J Parasitol. 2011 Jan;41(1):21-31. doi: 10.1016/j.ijpara.2010.06.008. Epub 2010 Aug 12. Int J Parasitol. 2011. PMID: 20708623
-
Parasite densities modulate susceptibility of mice to cerebral malaria during co-infection with Schistosoma japonicum and Plasmodium berghei.Malar J. 2014 Mar 26;13:116. doi: 10.1186/1475-2875-13-116. Malar J. 2014. PMID: 24670210 Free PMC article.
-
Schistosoma mansoni infection impairs antimalaria treatment and immune responses of rhesus macaques infected with mosquito-borne Plasmodium coatneyi.Infect Immun. 2012 Nov;80(11):3821-7. doi: 10.1128/IAI.00590-12. Epub 2012 Aug 20. Infect Immun. 2012. PMID: 22907811 Free PMC article.
-
Genetic analysis of cerebral malaria in the mouse model infected with Plasmodium berghei.Mamm Genome. 2018 Aug;29(7-8):488-506. doi: 10.1007/s00335-018-9752-9. Epub 2018 Jun 19. Mamm Genome. 2018. PMID: 29922917 Review.
-
Cerebral malaria: mysteries at the blood-brain barrier.Virulence. 2012 Mar-Apr;3(2):193-201. doi: 10.4161/viru.19013. Epub 2012 Mar 1. Virulence. 2012. PMID: 22460644 Free PMC article. Review.
Cited by
-
Maternal Schistosomiasis: Immunomodulatory Effects With Lasting Impact on Allergy and Vaccine Responses.Front Immunol. 2018 Dec 18;9:2960. doi: 10.3389/fimmu.2018.02960. eCollection 2018. Front Immunol. 2018. PMID: 30619318 Free PMC article. Review.
-
Infection against infection: parasite antagonism against parasites, viruses and bacteria.Infect Dis Poverty. 2019 Jun 15;8(1):49. doi: 10.1186/s40249-019-0560-6. Infect Dis Poverty. 2019. PMID: 31200765 Free PMC article. Review.
-
Single-cell RNA sequencing reveals a peripheral landscape of immune cells in Schistosomiasis japonica.Parasit Vectors. 2023 Oct 10;16(1):356. doi: 10.1186/s13071-023-05975-y. Parasit Vectors. 2023. PMID: 37817226 Free PMC article.
-
Modulation of paraoxonases during infectious diseases and its potential impact on atherosclerosis.Lipids Health Dis. 2012 Jul 23;11:92. doi: 10.1186/1476-511X-11-92. Lipids Health Dis. 2012. PMID: 22824324 Free PMC article. Review.
-
Day-to-day fluctuation of point-of-care circulating cathodic antigen test scores and faecal egg counts in children infected with Schistosoma mansoni in Ethiopia.BMC Infect Dis. 2014 Apr 17;14:210. doi: 10.1186/1471-2334-14-210. BMC Infect Dis. 2014. PMID: 24742192 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

