Residual gammaH2AX foci as an indication of lethal DNA lesions
- PMID: 20051134
- PMCID: PMC2819996
- DOI: 10.1186/1471-2407-10-4
Residual gammaH2AX foci as an indication of lethal DNA lesions
Abstract
Background: Evidence suggests that tumor cells exposed to some DNA damaging agents are more likely to die if they retain microscopically visible gammaH2AX foci that are known to mark sites of double-strand breaks. This appears to be true even after exposure to the alkylating agent MNNG that does not cause direct double-strand breaks but does produce gammaH2AX foci when damaged DNA undergoes replication.
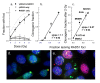
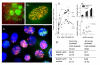
Methods: To examine this predictive ability further, SiHa human cervical carcinoma cells were exposed to 8 DNA damaging drugs (camptothecin, cisplatin, doxorubicin, etoposide, hydrogen peroxide, MNNG, temozolomide, and tirapazamine) and the fraction of cells that retained gammaH2AX foci 24 hours after a 30 or 60 min treatment was compared with the fraction of cells that lost clonogenicity. To determine if cells with residual repair foci are the cells that die, SiHa cervical cancer cells were stably transfected with a RAD51-GFP construct and live cell analysis was used to follow the fate of irradiated cells with RAD51-GFP foci.
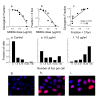
Results: For all drugs regardless of their mechanism of interaction with DNA, close to a 1:1 correlation was observed between clonogenic surviving fraction and the fraction of cells that retained gammaH2AX foci 24 hours after treatment. Initial studies established that the fraction of cells that retained RAD51 foci after irradiation was similar to the fraction of cells that retained gammaH2AX foci and subsequently lost clonogenicity. Tracking individual irradiated live cells confirmed that SiHa cells with RAD51-GFP foci 24 hours after irradiation were more likely to die.
Conclusion: Retention of DNA damage-induced gammaH2AX foci appears to be indicative of lethal DNA damage so that it may be possible to predict tumor cell killing by a wide variety of DNA damaging agents simply by scoring the fraction of cells that retain gammaH2AX foci.
Figures



Similar articles
-
Radiation sensitivity, H2AX phosphorylation, and kinetics of repair of DNA strand breaks in irradiated cervical cancer cell lines.Cancer Res. 2004 Oct 1;64(19):7144-9. doi: 10.1158/0008-5472.CAN-04-1433. Cancer Res. 2004. PMID: 15466212
-
Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks.Cancer Res. 2003 Aug 1;63(15):4347-50. Cancer Res. 2003. PMID: 12907603
-
Phosphorylated histone H2AX in spheroids, tumors, and tissues of mice exposed to etoposide and 3-amino-1,2,4-benzotriazine-1,3-dioxide.Cancer Res. 2004 Aug 1;64(15):5363-9. doi: 10.1158/0008-5472.CAN-04-0729. Cancer Res. 2004. PMID: 15289343
-
Retention of γH2AX foci as an indication of lethal DNA damage.Radiother Oncol. 2011 Oct;101(1):18-23. doi: 10.1016/j.radonc.2011.05.055. Epub 2011 Jun 23. Radiother Oncol. 2011. PMID: 21704409 Review.
-
DNA damage foci: Meaning and significance.Environ Mol Mutagen. 2015 Jul;56(6):491-504. doi: 10.1002/em.21944. Epub 2015 Mar 12. Environ Mol Mutagen. 2015. PMID: 25773265 Review.
Cited by
-
Changes in the number of double-strand DNA breaks in Chinese hamster V79 cells exposed to γ-radiation with different dose rates.Int J Mol Sci. 2013 Jul 1;14(7):13719-26. doi: 10.3390/ijms140713719. Int J Mol Sci. 2013. PMID: 23880845 Free PMC article.
-
MutS homologue hMSH5: role in cisplatin-induced DNA damage response.Mol Cancer. 2012 Mar 8;11:10. doi: 10.1186/1476-4598-11-10. Mol Cancer. 2012. PMID: 22401567 Free PMC article.
-
Exonuclease 1 (Exo1) is required for activating response to S(N)1 DNA methylating agents.DNA Repair (Amst). 2012 Dec 1;11(12):951-64. doi: 10.1016/j.dnarep.2012.09.004. Epub 2012 Oct 11. DNA Repair (Amst). 2012. PMID: 23062884 Free PMC article.
-
RNF8-independent Lys63 poly-ubiquitylation prevents genomic instability in response to replication-associated DNA damage.PLoS One. 2014 Feb 28;9(2):e89997. doi: 10.1371/journal.pone.0089997. eCollection 2014. PLoS One. 2014. PMID: 24587176 Free PMC article.
-
Mechanistic Modelling of Radiation Responses.Cancers (Basel). 2019 Feb 10;11(2):205. doi: 10.3390/cancers11020205. Cancers (Basel). 2019. PMID: 30744204 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

