Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study
- PMID: 20051135
- PMCID: PMC2822814
- DOI: 10.1186/1471-2466-10-1
Improved adherence with once-daily versus twice-daily dosing of mometasone furoate administered via a dry powder inhaler: a randomized open-label study
Abstract
Background: Poor adherence with prescribed asthma medication is a major barrier to positive treatment outcomes. This study was designed to determine the effect of a once-daily administration of mometasone furoate administered via a dry powder inhaler (MF-DPI) on treatment adherence compared with a twice-daily administration.
Methods: This was a 12-week open-label study designed to mimic an actual clinical setting in patients >or=12 years old with mild-to-moderate persistent asthma. Patients were randomized to receive MF-DPI 400 microg once-daily in the evening or MF-DPI 200 microg twice-daily. Adherence was assessed primarily using the number of actual administered doses reported from the device counter divided by the number of scheduled doses. Self-reports were also used to determine adherence. Health-related quality of life, healthcare resource utilization, and days missed from work or school were also reported.
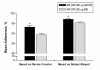
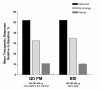
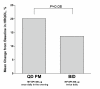
Results: 1233 patients were randomized. The mean adherence rates, as measured by the automatic dose counter, were significantly better (P < 0.001) with MF-DPI 400 microg once-daily in the evening (93.3%) than with MF-DPI 200 microg twice-daily (89.5%). Mean adherence rates based on self-reports were also significantly better (P < 0.001) with MF-DPI 400 microg QD PM (97.2%) than with MF-DPI 200 microg twice-daily (95.3%). Adherence rates were lower in adolescents (12-17 years old). Health-related quality of life improved by 20% in patients using MF-DPI once-daily in the evening and by 14% in patients using MF-DPI twice-daily. Very few (<8%) patients missed work/school.
Conclusion: Mean adherence rates were greater with a once-daily dosing regimen of MF-DPI than with a twice-daily dosing regimen.This trial was completed prior to the ISMJE requirements for trial registration.
Figures
References
-
- Global Strategy for Asthma Management and Prevention. http://www.ginasthma.com/Guidelineitem.asp??l1=2&l2=1&intId=60
-
- British guideline on the management of asthma: revised edition. http://www.brit-thoracic.org.uk/ClinicalInformation/Asthma/AsthmaGuideli...
-
- Chmelik F, Doughty A. Objective measurements of compliance in asthma treatment. Ann Allergy. 1994;73(6):527–532. - PubMed
-
- Onyirimba F, Apter A, Reisine S, Litt M, McCusker C, Connors M, ZuWallack R. Direct clinician-to-patient feedback discussion of inhaled steroid use: its effect on adherence. Ann Allergy Asthma Immunol. 2003;90(4):411–415. - PubMed
-
- Bender B, Milgrom H, Rand C. Nonadherence in asthmatic patients: is there a solution to the problem? Ann Allergy Asthma Immunol. 1997;79(3):177–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

