KChIP2 attenuates cardiac hypertrophy through regulation of Ito and intracellular calcium signaling
- PMID: 20051248
- PMCID: PMC2866822
- DOI: 10.1016/j.yjmcc.2009.12.019
KChIP2 attenuates cardiac hypertrophy through regulation of Ito and intracellular calcium signaling
Abstract
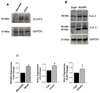
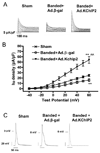
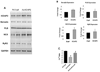
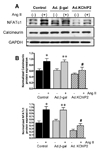
Recent evidence shows that the auxiliary subunit KChIP2, which assembles with pore-forming Kv4-subunits, represents a new potential regulator of the cardiac calcium-independent transient outward potassium current (I(to)) density. In hypertrophy and heart failure, KChIP2 expression has been found to be significantly decreased. Our aim was to examine the role of KChIP2 in cardiac hypertrophy and the effect of restoring its expression on electrical remodeling and cardiac mechanical function using a combination of molecular, biochemical and gene targeting approaches. KChIP2 overexpression through gene transfer of Ad.KChIP2 in neonatal cardiomyocytes resulted in a significant increase in I(to)-channel forming Kv4.2 and Kv4.3 protein levels. In vivo gene transfer of KChIP2 in aortic banded adult rats showed that, compared to sham-operated or Ad.beta-gal-transduced hearts, KChIP2 significantly attenuated the developed left ventricular hypertrophy, robustly increased I(to) densities, shortened action potential duration, and significantly altered myocyte mechanics by shortening contraction amplitudes and maximal rates of contraction and relaxation velocities and decreasing Ca(2+) transients. Interestingly, blocking I(to) with 4-aminopyridine in KChIP2-overexpressing adult cardiomyocytes significantly increased the Ca(2+) transients to control levels. One-day-old rat pups intracardially transduced with KChIP2 for two months then subjected to aortic banding for 6-8 weeks (to induce hypertrophy) showed similar echocardiographic, electrical and mechanical remodeling parameters. In addition, in cultured adult cardiomyocytes, KChIP2 overexpression increased the expression of Ca(2+)-ATPase (SERCA2a) and sodium calcium exchanger but had no effect on ryanodine receptor 2 or phospholamban expression. In neonatal myocytes, KChIP2 notably reversed Ang II-induced hypertrophic changes in protein synthesis and MAP-kinase activation. It also significantly decreased calcineurin expression, NFATc1 expression and nuclear translocation and its downstream target, MCiP1.4. Altogether, these data show that KChIP2 can attenuate cardiac hypertrophy possibly through modulation of intracellular calcium concentration and calcineurin/NFAT pathway.
(c) 2009. Published by Elsevier Ltd. All rights reserved.
Figures







References
-
- Nabauer M, Kaab S. Potassium channel down-regulation in heart failure. Cardiovascular research. 1998 Feb;37(2):324–334. - PubMed
-
- Tomaselli GF, Marban E. Electrophysiological remodeling in hypertrophy and heart failure. Cardiovascular research. 1999 May;42(2):270–283. - PubMed
-
- Wickenden AD, Kaprielian R, Kassiri Z, Tsoporis JN, Tsushima R, Fishman GI, et al. The role of action potential prolongation and altered intracellular calcium handling in the pathogenesis of heart failure. Cardiovascular research. 1998 Feb;37(2):312–323. - PubMed
-
- Gidh-Jain M, Huang B, Jain P, el-Sherif N. Differential expression of voltage-gated K+ channel genes in left ventricular remodeled myocardium after experimental myocardial infarction. Circulation research. 1996 Oct;79(4):669–675. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

