Phase 1 safety and immunogenicity trial of the Plasmodium falciparum blood-stage malaria vaccine AMA1-C1/ISA 720 in Australian adults
- PMID: 20051276
- PMCID: PMC2847846
- DOI: 10.1016/j.vaccine.2009.12.049
Phase 1 safety and immunogenicity trial of the Plasmodium falciparum blood-stage malaria vaccine AMA1-C1/ISA 720 in Australian adults
Abstract
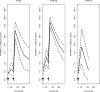
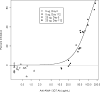
A Phase 1 trial was conducted in malaria-naïve adults to evaluate the recombinant protein vaccine apical membrane antigen 1-Combination 1 (AMA1-C1) formulated in Montanide ISA 720 (SEPPIC, France), a water-in-oil adjuvant. Vaccinations were halted early due to a formulation issue unrelated to stability or potency. Twenty-four subjects (12 in each group) were enrolled and received 5 or 20 microg protein at 0 and 3 months and four subjects were enrolled and received one vaccination of 80 microg protein. After first vaccination, nearly all subjects experienced mild to moderate local reactions and six experienced delayed local reactions occurring at Day 9 or later. After the second vaccination, three subjects experienced transient grade 3 (severe) local reactions; the remainder experienced grade 1 or 2 local reactions. All related systemic reactogenicity was grade 1 or 2, except one instance of grade 3 malaise. Anti-AMA1-C1 antibody responses were dose dependent and seen following each vaccination, with mean antibody levels 2-3 fold higher in the 20 microg group compared to the 5 microg group at most time points. In vitro growth-inhibitory activity was a function of the anti-AMA1 antibody titer. AMA1-C1 formulated in ISA 720 is immunogenic in malaria-naïve Australian adults. It is reasonably tolerated, though some transient, severe, and late local reactions are seen.
Published by Elsevier Ltd.
Figures
Similar articles
-
Long term stability of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Montanide(®) ISA 720 and stabilized with glycine.Vaccine. 2011 May 9;29(20):3640-5. doi: 10.1016/j.vaccine.2011.03.015. Epub 2011 Apr 8. Vaccine. 2011. PMID: 21440641 Free PMC article.
-
Safety and immunogenicity of multi-antigen AMA1-based vaccines formulated with CoVaccine HT™ and Montanide ISA 51 in rhesus macaques.Malar J. 2011 Jul 4;10:182. doi: 10.1186/1475-2875-10-182. Malar J. 2011. PMID: 21726452 Free PMC article.
-
Phase I Clinical Trial of a Recombinant Blood Stage Vaccine Candidate for Plasmodium falciparum Malaria Based on MSP1 and EBA175.PLoS One. 2015 Apr 30;10(4):e0117820. doi: 10.1371/journal.pone.0117820. eCollection 2015. PLoS One. 2015. PMID: 25927360 Free PMC article. Clinical Trial.
-
A human phase 1 vaccine clinical trial of the Plasmodium falciparum malaria vaccine candidate apical membrane antigen 1 in Montanide ISA720 adjuvant.Vaccine. 2005 Apr 27;23(23):3076-83. doi: 10.1016/j.vaccine.2004.09.040. Vaccine. 2005. PMID: 15811655 Clinical Trial.
-
Safety and immunogenicity of a recombinant Plasmodium falciparum AMA1 malaria vaccine adjuvanted with Alhydrogel, Montanide ISA 720 or AS02.PLoS One. 2008;3(12):e3960. doi: 10.1371/journal.pone.0003960. Epub 2008 Dec 18. PLoS One. 2008. PMID: 19093004 Free PMC article. Clinical Trial.
Cited by
-
Malaria vaccine adjuvants: latest update and challenges in preclinical and clinical research.Biomed Res Int. 2013;2013:282913. doi: 10.1155/2013/282913. Epub 2013 Apr 23. Biomed Res Int. 2013. PMID: 23710439 Free PMC article. Review.
-
Blood stage vaccines for Plasmodium falciparum: current status and the way forward.Hum Vaccin. 2010 Aug;6(8):627-34. doi: 10.4161/hv.6.8.11446. Hum Vaccin. 2010. PMID: 20519960 Free PMC article. Review.
-
Adenovirus 5-vectored P. falciparum vaccine expressing CSP and AMA1. Part A: safety and immunogenicity in seronegative adults.PLoS One. 2011;6(10):e24586. doi: 10.1371/journal.pone.0024586. Epub 2011 Oct 7. PLoS One. 2011. PMID: 22003383 Free PMC article. Clinical Trial.
-
Combining viral vectored and protein-in-adjuvant vaccines against the blood-stage malaria antigen AMA1: report on a phase 1a clinical trial.Mol Ther. 2014 Dec;22(12):2142-2154. doi: 10.1038/mt.2014.157. Epub 2014 Aug 26. Mol Ther. 2014. PMID: 25156127 Free PMC article. Clinical Trial.
-
Immunization with the Malaria Diversity-Covering Blood-Stage Vaccine Candidate Plasmodium falciparum Apical Membrane Antigen 1 DiCo in Complex with Its Natural Ligand PfRon2 Does Not Improve the In Vitro Efficacy.Front Immunol. 2017 Jun 27;8:743. doi: 10.3389/fimmu.2017.00743. eCollection 2017. Front Immunol. 2017. PMID: 28702028 Free PMC article.
References
-
- WHO. World Malaria Report. 2008. http://www.who.int/malaria/wmr2008/malaria2008.pdf
-
- Remarque EJ, Faber BW, Kocken CH, Thomas AW. Apical membrane antigen 1: a malaria vaccine candidate in review. Trends in Parasitology. 2008;24(2):74–84. - PubMed
-
- Kennedy MC, Wang J, Zhang Y, Miles AP, Chitsaz F, Saul A, et al. In vitro studies with recombinant Plasmodium falciparum apical membrane antigen 1 (AMA1): production and activity of an AMA1 vaccine and generation of a multiallelic response. Infection and immunity. 2002;70(12):6948–6960. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

