Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence
- PMID: 20051379
- PMCID: PMC2803700
- DOI: 10.1158/1940-6207.CAPR-09-0059
Atorvastatin and celecoxib in combination inhibits the progression of androgen-dependent LNCaP xenograft prostate tumors to androgen independence
Abstract
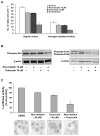
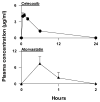
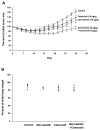
Epidemiology studies suggest that statins and nonsteroidal anti-inflammatory drugs reduce the risk of prostate cancer. In the present study, LNCaP cells were cultured in regular medium containing fetal bovine serum or in medium supplemented with charcoal-stripped fetal bovine serum to mimic androgen deprivation treatment. We found that atorvastatin (Lipitor) or celecoxib (Celebrex) treatment of LNCaP cells cultured in regular or androgen-depleted medium inhibited growth and stimulated apoptosis. A combination of atorvastatin and celecoxib was more effective than either agent alone. In animal studies, severe combined immunodeficient mice were injected s.c. with LNCaP cells in Matrigel. After 4 to 6 weeks, mice with LNCaP tumors (about 0.6 cm wide and 0.6 cm long) were surgically castrated and received daily i.p. injections of vehicle, atorvastatin (10 microg/g body weight/d), celecoxib (10 microg/g/d), or a combination of atorvastatin (5 microg/g/d) and celecoxib (5 microg/g/d) for 42 days. In all groups, the androgen-dependent LNCaP tumors regressed initially in response to castration, but the tumors eventually progressed to androgen independence and started to grow. Treatment of the mice with atorvastatin or celecoxib alone suppressed the regrowth of LNCaP tumors after castration. A combination of low doses of atorvastatin and celecoxib had a more potent effect in inhibiting the growth and progression of LNCaP tumors to androgen independence than a higher dose of either agent alone. Our results indicate that administration of a combination of atorvastatin and celecoxib may be an effective strategy for the prevention of prostate cancer progression from androgen dependence to androgen independence.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics. CA Cancer J Clin. 2008;58(2):71–96. - PubMed
-
- Morris MJ, Scher HI. Novel therapies for the treatment of prostate cancer: current clinical trials and development stratagies. Surg Oncol. 2002;11:13–23. - PubMed
-
- So A, Gleave M, Hurtado-Col A, Nelson C. Mechanisms of the development of androgen independence in prostate cancer. World J Urol. 2005;23(1):1–9. - PubMed
-
- Pilat MJ, Kamradt JM, Pienta KJ. Hormone resistance in prostate cancer. Cancer Metastasis Rev. 1998;17(4):373–81. - PubMed
-
- Raghavan D, Koczwara B, Javle M. Evolving strategies of cytotoxic chemotherapy for advanced prostate cancer. Eur J Cancer. 1997;33(4):566–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

