RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle
- PMID: 20051487
- PMCID: PMC2817627
- DOI: 10.1210/en.2009-0978
RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle
Abstract
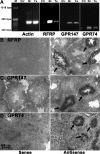
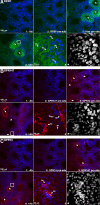
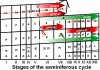
RFamide-related peptide (RFRP), the mammalian homolog of avian gonadotropin-inhibitory hormone, has a pronounced suppressive action on the reproductive axis across species. In mammals, RFRP acts directly on GnRH neurons, and likely at the level of the pituitary, to inhibit gonadotropin secretion. In the present study, we examined whether RFRP might act outside of mammalian brain on reproductive tissues directly. Using RT-PCR and in situ hybridization, we found that both RFRP and its receptors [G protein-coupled receptor (GPR) 147 and GPR74] are expressed in the testis of Syrian hamster. These results were confirmed and extended using double- and triple-label immunohistochemistry. RFRP expression was observed in spermatocytes and in round to early elongated spermatids. Significant expression of RFRP was not seen in Leydig cells. GPR147 protein was observed in myoid cells in all stages of spermatogenesis, pachytene spermatocytes, maturation division spermatocytes, and in round and late elongated spermatids. GPR74 proteins only appeared in late elongated spermatids. Additionally, we found that RFRP and its receptor mRNA are markedly altered by day length and reproductive condition. These findings highlight a possible novel autocrine and/or paracrine role for RFRP in Syrian hamster testis, potentially contributing to the differentiation of spermatids during spermiogenesis.
Figures



References
-
- Castellano JM, Gaytan M, Roa J, Vigo E, Navarro VM, Bellido C, Dieguez C, Aguilar E, Sánchez-Criado JE, Pellicer A, Pinilla L, Gaytan F, Tena-Sempere M 2006 Expression of KiSS-1 in rat ovary: putative local regulator of ovulation? Endocrinology 147:4852–4862 - PubMed
-
- Maddineni SR, Ocón-Grove OM, Krzysik-Walker SM, Hendricks 3rd GL, Ramachandran R 2008 Gonadotropin-inhibitory hormone (GnIH) receptor gene is expressed in the chicken ovary: potential role of GnIH in follicular maturation. Reproduction 135:267–274 - PubMed
-
- Bentley GE, Ubuka T, McGuire NL, Chowdhury VS, Morita Y, Yano T, Hasunuma I, Binns M, Wingfield JC, Tsutsui K 2008 Gonadotropin-inhibitory hormone and its receptor in the avian reproductive system. Gen Comp Endocrinol 156:34–43 - PubMed

