Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport
- PMID: 20051516
- PMCID: PMC2844166
- DOI: 10.1074/jbc.M109.047829
Disruption of the K+ channel beta-subunit KCNE3 reveals an important role in intestinal and tracheal Cl- transport
Abstract
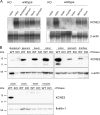
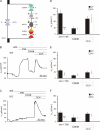
The KCNE3 beta-subunit constitutively opens outwardly rectifying KCNQ1 (Kv7.1) K(+) channels by abolishing their voltage-dependent gating. The resulting KCNQ1/KCNE3 heteromers display enhanced sensitivity to K(+) channel inhibitors like chromanol 293B. KCNE3 was also suggested to modify biophysical properties of several other K(+) channels, and a mutation in KCNE3 was proposed to underlie forms of human periodic paralysis. To investigate physiological roles of KCNE3, we now disrupted its gene in mice. kcne3(-/-) mice were viable and fertile and displayed neither periodic paralysis nor other obvious skeletal muscle abnormalities. KCNQ1/KCNE3 heteromers are present in basolateral membranes of intestinal and tracheal epithelial cells where they might facilitate transepithelial Cl(-) secretion through basolateral recycling of K(+) ions and by increasing the electrochemical driving force for apical Cl(-) exit. Indeed, cAMP-stimulated electrogenic Cl(-) secretion across tracheal and intestinal epithelia was drastically reduced in kcne3(-/-) mice. Because the abundance and subcellular localization of KCNQ1 was unchanged in kcne3(-/-) mice, the modification of biophysical properties of KCNQ1 by KCNE3 is essential for its role in intestinal and tracheal transport. Further, these results suggest KCNE3 as a potential modifier gene in cystic fibrosis.
Figures





Similar articles
-
K2P TASK-2 and KCNQ1-KCNE3 K+ channels are major players contributing to intestinal anion and fluid secretion.J Physiol. 2018 Feb 1;596(3):393-407. doi: 10.1113/JP275178. Epub 2017 Dec 18. J Physiol. 2018. PMID: 29143340 Free PMC article.
-
Sexual dimorphism and oestrogen regulation of KCNE3 expression modulates the functional properties of KCNQ1 K⁺ channels.J Physiol. 2011 Nov 1;589(Pt 21):5091-107. doi: 10.1113/jphysiol.2011.215772. Epub 2011 Sep 12. J Physiol. 2011. PMID: 21911611 Free PMC article.
-
The small conductance K+ channel, KCNQ1: expression, function, and subunit composition in murine trachea.J Biol Chem. 2001 Nov 9;276(45):42268-75. doi: 10.1074/jbc.M105014200. Epub 2001 Aug 29. J Biol Chem. 2001. PMID: 11527966
-
The very small-conductance K+ channel KvLQT1 and epithelial function.Pflugers Arch. 2000 Jun;440(2):202-6. doi: 10.1007/s004240000257. Pflugers Arch. 2000. PMID: 10898519 Review.
-
KCNE1 and KCNE3: The yin and yang of voltage-gated K(+) channel regulation.Gene. 2016 Jan 15;576(1 Pt 1):1-13. doi: 10.1016/j.gene.2015.09.059. Epub 2015 Sep 26. Gene. 2016. PMID: 26410412 Free PMC article. Review.
Cited by
-
Opiate-induced constipation related to activation of small intestine opioid μ2-receptors.World J Gastroenterol. 2012 Mar 28;18(12):1391-6. doi: 10.3748/wjg.v18.i12.1391. World J Gastroenterol. 2012. PMID: 22493554 Free PMC article.
-
Novel exon 1 protein-coding regions N-terminally extend human KCNE3 and KCNE4.FASEB J. 2016 Aug;30(8):2959-69. doi: 10.1096/fj.201600467R. Epub 2016 May 9. FASEB J. 2016. PMID: 27162025 Free PMC article.
-
Allergic sensitization enhances anion current responsiveness of murine trachea to PAR-2 activation.Pflugers Arch. 2012 Mar;463(3):497-509. doi: 10.1007/s00424-011-1064-9. Epub 2011 Dec 15. Pflugers Arch. 2012. PMID: 22170096
-
Targeted deletion of Kcne3 impairs skeletal muscle function in mice.FASEB J. 2017 Jul;31(7):2937-2947. doi: 10.1096/fj.201600965RR. Epub 2017 Mar 29. FASEB J. 2017. PMID: 28356343 Free PMC article.
-
KCNE2 and the K (+) channel: the tail wagging the dog.Channels (Austin). 2012 Jan-Feb;6(1):1-10. doi: 10.4161/chan.19126. Epub 2012 Jan 1. Channels (Austin). 2012. PMID: 22513486 Free PMC article. Review.
References
-
- Takumi T., Ohkubo H., Nakanishi S. (1988) Science 242, 1042–1045 - PubMed
-
- Aguilar-Bryan L., Nichols C. G., Wechsler S. W., Clement J. P., Boyd A. E., 3rd, González G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D. A. (1995) Science 268, 423–426 - PubMed
-
- Sanguinetti M. C. (2000) Trends Pharmacol. Sci. 21, 199–201 - PubMed
-
- McCrossan Z. A., Abbott G. W. (2004) Neuropharmacology 47, 787–821 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

