The anti-apoptotic protein BCL2L1/Bcl-xL is neutralized by pro-apoptotic PMAIP1/Noxa in neuroblastoma, thereby determining bortezomib sensitivity independent of prosurvival MCL1 expression
- PMID: 20051518
- PMCID: PMC2844140
- DOI: 10.1074/jbc.M109.038331
The anti-apoptotic protein BCL2L1/Bcl-xL is neutralized by pro-apoptotic PMAIP1/Noxa in neuroblastoma, thereby determining bortezomib sensitivity independent of prosurvival MCL1 expression
Abstract
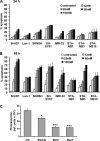
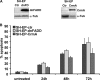
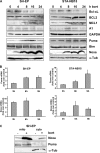
Neuroblastoma is the most frequent extracranial solid tumor in children. Here, we report that the proteasome inhibitor bortezomib (PS-341, Velcade) activated the pro-apoptotic BH3-only proteins PMAIP1/Noxa and BBC3/Puma and induced accumulation of anti-apoptotic MCL1 as well as repression of anti-apoptotic BCL2L1/Bcl-xL. Retroviral expression of Bcl-xL, but not of MCL1, prevented apoptosis by bortezomib. Gene knockdown of Noxa by shRNA technology significantly reduced apoptosis, whereas Puma knockdown did not affect cell death kinetics. Immunoprecipitation revealed that endogenous Noxa associated with both, Bcl-xL and MCL1, suggesting that in neuronal cells Noxa can neutralize Bcl-xL, explaining the pronounced protective effect of Bcl-xL. Tetracycline-regulated Noxa expression did not trigger cell death per se but sensitized to bortezomib treatment in a dose-dependent manner. This implies that the induction of Noxa is necessary but not sufficient for bortezomib-induced apoptosis. We conclude that MCL1 steady-state expression levels do not affect sensitivity to proteasome-inhibitor treatment in neuronal tumor cells, and that both the repression of Bcl-xL and the activation of Noxa are necessary for bortezomib-induced cell death.
Figures
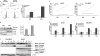

Similar articles
-
Proteasome inhibition induces apoptosis through simultaneous inactivation of MCL-1/BCL-XL by NOXA independent of CHOP and JNK pathways.Toxicology. 2024 Nov;508:153906. doi: 10.1016/j.tox.2024.153906. Epub 2024 Aug 6. Toxicology. 2024. PMID: 39117261
-
Bcl-2 is a better ABT-737 target than Bcl-xL or Bcl-w and only Noxa overcomes resistance mediated by Mcl-1, Bfl-1, or Bcl-B.Cell Death Dis. 2012 Aug 9;3(8):e366. doi: 10.1038/cddis.2012.109. Cell Death Dis. 2012. PMID: 22875003 Free PMC article.
-
Noxa up-regulation and Mcl-1 cleavage are associated to apoptosis induction by bortezomib in multiple myeloma.Cancer Res. 2007 Jun 1;67(11):5418-24. doi: 10.1158/0008-5472.CAN-06-4322. Cancer Res. 2007. PMID: 17545623
-
Altering protein turnover in tumor cells: new opportunities for anti-cancer therapies.Drug Resist Updat. 2005 Dec;8(6):359-68. doi: 10.1016/j.drup.2005.12.001. Epub 2006 Jan 6. Drug Resist Updat. 2005. PMID: 16406769 Review.
-
Bortezomib as the first proteasome inhibitor anticancer drug: current status and future perspectives.Curr Cancer Drug Targets. 2011 Mar;11(3):239-53. doi: 10.2174/156800911794519752. Curr Cancer Drug Targets. 2011. PMID: 21247388 Free PMC article. Review.
Cited by
-
Therapy-resistant acute lymphoblastic leukemia (ALL) cells inactivate FOXO3 to escape apoptosis induction by TRAIL and Noxa.Oncotarget. 2013 Jul;4(7):995-1007. doi: 10.18632/oncotarget.953. Oncotarget. 2013. PMID: 23828551 Free PMC article.
-
BCL2 Inhibitors as Anticancer Drugs: A Plethora of Misleading BH3 Mimetics.Mol Cancer Ther. 2016 Sep;15(9):2011-7. doi: 10.1158/1535-7163.MCT-16-0031. Epub 2016 Aug 17. Mol Cancer Ther. 2016. PMID: 27535975 Free PMC article. Review.
-
Targeting neuroblastoma stem cells with retinoic acid and proteasome inhibitor.PLoS One. 2013 Oct 7;8(10):e76761. doi: 10.1371/journal.pone.0076761. eCollection 2013. PLoS One. 2013. PMID: 24116151 Free PMC article.
-
BRAF associated autophagy exploitation: BRAF and autophagy inhibitors synergise to efficiently overcome resistance of BRAF mutant colorectal cancer cells.Oncotarget. 2016 Feb 23;7(8):9188-221. doi: 10.18632/oncotarget.6942. Oncotarget. 2016. PMID: 26802026 Free PMC article.
-
Multiple BH3 mimetics antagonize antiapoptotic MCL1 protein by inducing the endoplasmic reticulum stress response and up-regulating BH3-only protein NOXA.J Biol Chem. 2011 Jul 15;286(28):24882-95. doi: 10.1074/jbc.M111.255828. Epub 2011 May 31. J Biol Chem. 2011. PMID: 21628457 Free PMC article.
References
-
- Park J. R., Eggert A., Caron H. (2008) Pediatr. Clin. North Am. 55, 97–120 - PubMed
-
- Adams J. (2002) Breast Dis. 15, 61–70 - PubMed
-
- Jagannath S., Barlogie B., Berenson J., Siegel D., Irwin D., Richardson P. G., Niesvizky R., Alexanian R., Limentani S. A., Alsina M., Adams J., Kauffman M., Esseltine D. L., Schenkein D. P., Anderson K. C. (2004) Br. J. Haematol. 127, 165–172 - PubMed
-
- Blaney S. M., Bernstein M., Neville K., Ginsberg J., Kitchen B., Horton T., Berg S. L., Krailo M., Adamson P. C. (2004) J. Clin. Oncol. 22, 4804–4809 - PubMed
-
- Strasser A. (2005) Nat. Rev. Immunol. 5, 189–200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

