Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias
- PMID: 20051519
- PMCID: PMC2844194
- DOI: 10.1074/jbc.M109.094011
Identification of orthosteric and allosteric site mutations in M2 muscarinic acetylcholine receptors that contribute to ligand-selective signaling bias
Abstract
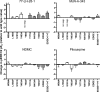
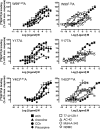
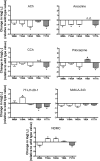
Muscarinic acetylcholine receptors contain at least one allosteric site that is topographically distinct from the acetylcholine, orthosteric binding site. Although studies have investigated the basis of allosteric modulation at these receptors, less is known about putative allosteric ligands that activate the receptor in their own right. We generated M(2) muscarinic acetylcholine receptor mutations in either the orthosteric site in transmembrane helices 3 and 6 (TM3 and -6) or part of an allosteric site involving the top of TM2, the second extracellular (E2) loop, and the top of TM7 and investigated their effects on the binding and function of the novel selective (putative allosteric) agonists (AC-42 (4-n-butyl-1-(4-(2-methylphenyl)-4-oxo-1-butyl)piperidine HCl), 77-LH-28-1 (1-(3-(4-butyl-1-piperidinyl)propyl)-3,3-dihydro-2(1H)-quinolinone), and N-desmethylclozapine) as well as the bitopic orthosteric/allosteric ligand, McN-A-343 (4-(m-chlorophenyl-carbamoyloxy)-2-butynyltrimethylammonium). Four classes of agonists were identified, depending on their response to the mutations, suggesting multiple, distinct modes of agonist-receptor interaction. Interestingly, with the exception of 77-LH-28-1, allosteric site mutations had no effect on the affinity of any of the agonists tested, but some mutations in the E2 loop influenced the efficacy of both orthosteric and novel selective agonists, highlighting a role for this region of the receptor in modulating activation status. Two point mutations (Y104(3.33)A (Ballesteros and Weinstein numbers in superscript) in the orthosteric and Y177A in the allosteric site) unmasked ligand-selective and signaling pathway-selective effects, providing evidence for the existence of pathway-specific receptor conformations. Molecular modeling of 77-LH-28-1 and N-desmethylclozapine yielded novel binding poses consistent with the possibility that the functional selectivity of such agents may arise from a bitopic mechanism.
Figures





Similar articles
-
G protein coupling and signaling pathway activation by m1 muscarinic acetylcholine receptor orthosteric and allosteric agonists.J Pharmacol Exp Ther. 2008 Nov;327(2):365-74. doi: 10.1124/jpet.108.141788. Epub 2008 Jul 29. J Pharmacol Exp Ther. 2008. PMID: 18664591
-
Structure-function studies of allosteric agonism at M2 muscarinic acetylcholine receptors.Mol Pharmacol. 2007 Aug;72(2):463-76. doi: 10.1124/mol.107.037630. Epub 2007 May 24. Mol Pharmacol. 2007. PMID: 17525129
-
Orthosteric and allosteric modes of interaction of novel selective agonists of the M1 muscarinic acetylcholine receptor.Mol Pharmacol. 2010 Jul;78(1):94-104. doi: 10.1124/mol.110.064345. Epub 2010 Apr 22. Mol Pharmacol. 2010. PMID: 20413650
-
Development of allosteric modulators of GPCRs for treatment of CNS disorders.Neurobiol Dis. 2014 Jan;61:55-71. doi: 10.1016/j.nbd.2013.09.013. Epub 2013 Sep 27. Neurobiol Dis. 2014. PMID: 24076101 Free PMC article. Review.
-
Regulation of signal transduction at M2 muscarinic receptor.Physiol Res. 2004;53 Suppl 1:S131-40. Physiol Res. 2004. PMID: 15119944 Review.
Cited by
-
Structural determinants of allosteric agonism and modulation at the M4 muscarinic acetylcholine receptor: identification of ligand-specific and global activation mechanisms.J Biol Chem. 2010 Jun 18;285(25):19012-21. doi: 10.1074/jbc.M110.125096. Epub 2010 Apr 20. J Biol Chem. 2010. PMID: 20406819 Free PMC article.
-
Novel GPCR paradigms at the μ-opioid receptor.Br J Pharmacol. 2015 Jan;172(2):287-96. doi: 10.1111/bph.12600. Epub 2014 Jul 1. Br J Pharmacol. 2015. PMID: 24460711 Free PMC article. Review.
-
Human Neuropeptide S Receptor Is Activated via a Gαq Protein-biased Signaling Cascade by a Human Neuropeptide S Analog Lacking the C-terminal 10 Residues.J Biol Chem. 2016 Apr 1;291(14):7505-16. doi: 10.1074/jbc.M115.704122. Epub 2016 Feb 10. J Biol Chem. 2016. PMID: 26865629 Free PMC article.
-
Crystal structure of the α1B-adrenergic receptor reveals molecular determinants of selective ligand recognition.Nat Commun. 2022 Jan 19;13(1):382. doi: 10.1038/s41467-021-27911-3. Nat Commun. 2022. PMID: 35046410 Free PMC article.
-
A Receptor Model With Binding Affinity, Activation Efficacy, and Signal Amplification Parameters for Complex Fractional Response Versus Occupancy Data.Front Pharmacol. 2019 Jun 11;10:605. doi: 10.3389/fphar.2019.00605. eCollection 2019. Front Pharmacol. 2019. PMID: 31244653 Free PMC article.
References
-
- Eglen R. M., Choppin A., Watson N. (2001) Trends Pharmacol. Sci. 22, 409–414 - PubMed
-
- Wess J., Eglen R. M., Gautam D. (2007) Nat. Rev. Drug Discover. 6, 721–733 - PubMed
-
- Blüml K., Mutschler E., Wess J. (1994) J. Biol. Chem. 269, 18870–18876 - PubMed
-
- Spalding T. A., Burstein E. S., Henderson S. C., Ducote K. R., Brann M. R. (1998) J. Biol. Chem. 273, 21563–21568 - PubMed
-
- Heitz F., Holzwarth J. A., Gies J. P., Pruss R. M., Trumpp-Kallmeyer S., Hibert M. F., Guenet C. (1999) Eur. J. Pharmacol. 380, 183–195 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

