From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis
- PMID: 20051526
- PMCID: PMC3365845
- DOI: 10.1210/er.2009-0024
From estrogen-centric to aging and oxidative stress: a revised perspective of the pathogenesis of osteoporosis
Abstract
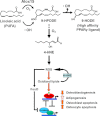
Estrogen deficiency has been considered the seminal mechanism of osteoporosis in both women and men, but epidemiological evidence in humans and recent mechanistic studies in rodents indicate that aging and the associated increase in reactive oxygen species (ROS) are the proximal culprits. ROS greatly influence the generation and survival of osteoclasts, osteoblasts, and osteocytes. Moreover, oxidative defense by the FoxO transcription factors is indispensable for skeletal homeostasis at any age. Loss of estrogens or androgens decreases defense against oxidative stress in bone, and this accounts for the increased bone resorption associated with the acute loss of these hormones. ROS-activated FoxOs in early mesenchymal progenitors also divert ss-catenin away from Wnt signaling, leading to decreased osteoblastogenesis. This latter mechanism may be implicated in the pathogenesis of type 1 and 2 diabetes and ROS-mediated adverse effects of diabetes on bone formation. Attenuation of Wnt signaling by the activation of peroxisome proliferator-activated receptor gamma by ligands generated from lipid oxidation also contributes to the age-dependent decrease in bone formation, suggesting a mechanistic explanation for the link between atherosclerosis and osteoporosis. Additionally, increased glucocorticoid production and sensitivity with advancing age decrease skeletal hydration and thereby increase skeletal fragility by attenuating the volume of the bone vasculature and interstitial fluid. This emerging evidence provides a paradigm shift from the "estrogen-centric" account of the pathogenesis of involutional osteoporosis to one in which age-related mechanisms intrinsic to bone and oxidative stress are protagonists and age-related changes in other organs and tissues, such as ovaries, accentuate them.
Figures



References
-
- Manolagas SC 2000 Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21:115–137 - PubMed
-
- Riggs BL, Khosla S, Melton 3rd LJ 2002 Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23:279–302 - PubMed
-
- Nordin BEC, Need AG, Prince RL, Horowitz M, Gutteridge DH, Papapoulos SE 1993 Osteoporosis. In: Nordin BEC, Need AG, Morris HA, eds. Metabolic bone and stone disease. Edinburgh, UK: Churchill Livingstone; 1–82
-
- Riggs BL, Khosla S, Melton 3rd LJ 1998 A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res 13:763–773 - PubMed
-
- Khosla S, Melton 3rd LJ, Atkinson EJ, O'Fallon WM, Klee GG, Riggs BL 1998 Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J Clin Endocrinol Metab 83:2266–2274 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

