Human carboxylesterase 1 stereoselectively binds the nerve agent cyclosarin and spontaneously hydrolyzes the nerve agent sarin
- PMID: 20051531
- PMCID: PMC2845941
- DOI: 10.1124/mol.109.062356
Human carboxylesterase 1 stereoselectively binds the nerve agent cyclosarin and spontaneously hydrolyzes the nerve agent sarin
Abstract
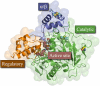
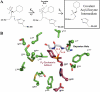
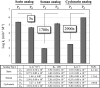
Organophosphorus (OP) nerve agents are potent toxins that inhibit cholinesterases and produce a rapid and lethal cholinergic crisis. Development of protein-based therapeutics is being pursued with the goal of preventing nerve agent toxicity and protecting against the long-term side effects of these agents. The drug-metabolizing enzyme human carboxylesterase 1 (hCE1) is a candidate protein-based therapeutic because of its similarity in structure and function to the cholinesterase targets of nerve agent poisoning. However, the ability of wild-type hCE1 to process the G-type nerve agents sarin and cyclosarin has not been determined. We report the crystal structure of hCE1 in complex with the nerve agent cyclosarin. We further use stereoselective nerve agent analogs to establish that hCE1 exhibits a 1700- and 2900-fold preference for the P(R) enantiomers of analogs of soman and cyclosarin, respectively, and a 5-fold preference for the P(S) isomer of a sarin analog. Finally, we show that for enzyme inhibited by racemic mixtures of bona fide nerve agents, hCE1 spontaneously reactivates in the presence of sarin but not soman or cyclosarin. The addition of the neutral oxime 2,3-butanedione monoxime increases the rate of reactivation of hCE1 from sarin inhibition by more than 60-fold but has no effect on reactivation with the other agents examined. Taken together, these data demonstrate that hCE1 is only reactivated after inhibition with the more toxic P(S) isomer of sarin. These results provide important insights toward the long-term goal of designing novel forms of hCE1 to act as protein-based therapeutics for nerve agent detoxification.
Figures




Similar articles
-
Crystal structures of human carboxylesterase 1 in covalent complexes with the chemical warfare agents soman and tabun.Biochemistry. 2007 May 1;46(17):5063-71. doi: 10.1021/bi700246n. Epub 2007 Apr 4. Biochemistry. 2007. PMID: 17407327 Free PMC article.
-
Crystal structures of brain group-VIII phospholipase A2 in nonaged complexes with the organophosphorus nerve agents soman and sarin.Biochemistry. 2009 Apr 21;48(15):3425-35. doi: 10.1021/bi8023527. Biochemistry. 2009. PMID: 19271773 Free PMC article.
-
Crystal structures of human group-VIIA phospholipase A2 inhibited by organophosphorus nerve agents exhibit non-aged complexes.Biochem Pharmacol. 2009 Aug 15;78(4):420-9. doi: 10.1016/j.bcp.2009.04.018. Epub 2009 Apr 24. Biochem Pharmacol. 2009. PMID: 19394314 Free PMC article.
-
Recent advances in evaluation of oxime efficacy in nerve agent poisoning by in vitro analysis.Toxicol Appl Pharmacol. 2007 Mar;219(2-3):226-34. doi: 10.1016/j.taap.2006.10.001. Epub 2006 Oct 6. Toxicol Appl Pharmacol. 2007. PMID: 17112559 Review.
-
The role of oximes in the treatment of nerve agent poisoning in civilian casualties.Toxicol Rev. 2006;25(4):297-323. doi: 10.2165/00139709-200625040-00009. Toxicol Rev. 2006. PMID: 17288500 Review.
Cited by
-
Interaction of the serine hydrolase KIAA1363 with organophosphorus agents: Evaluation of potency and kinetics.Arch Biochem Biophys. 2016 Jan 15;590:72-81. doi: 10.1016/j.abb.2015.11.034. Epub 2015 Nov 23. Arch Biochem Biophys. 2016. PMID: 26617293 Free PMC article.
-
Nanomaterial-Enabled Sensors and Therapeutic Platforms for Reactive Organophosphates.Nanomaterials (Basel). 2021 Jan 16;11(1):224. doi: 10.3390/nano11010224. Nanomaterials (Basel). 2021. PMID: 33467113 Free PMC article. Review.
-
In Silico Design and Evaluation of Carboxylesterase Inhibitors.J Pest Sci (2004). 2010;35(3):240-249. doi: 10.1584/jpestics.R10-06. J Pest Sci (2004). 2010. PMID: 23487487 Free PMC article.
-
Structure of recombinant human carboxylesterase 1 isolated from whole cabbage looper larvae.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Mar 1;68(Pt 3):269-72. doi: 10.1107/S1744309112003326. Epub 2012 Feb 15. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22442219 Free PMC article.
-
Breed Differences in Pig Liver Esterase (PLE) between Tongcheng (Chinese Local Breed) and Large White Pigs.Sci Rep. 2018 Nov 5;8(1):16364. doi: 10.1038/s41598-018-34695-y. Sci Rep. 2018. PMID: 30397234 Free PMC article.
References
-
- Aurbek N, Thiermann H, Szinicz L, Eyer P, Worek F. (2006) Analysis of inhibition, reactivation and aging kinetics of highly toxic organophosphorus compounds with human and pig acetylcholinesterase. Toxicology 224:91–99 - PubMed
-
- Bartling A, Worek F, Szinicz L, Thiermann H. (2007) Enzyme-kinetic investigation of different sarin analogues reacting with human acetylcholinesterase and butyrylcholinesterase. Toxicology 233:166–172 - PubMed
-
- Bencharit S, Morton CL, Howard-Williams EL, Danks MK, Potter PM, Redinbo MR. (2002) Structural insights into CPT-11 activation by mammalian carboxylesterases. Nat Struct Biol 9:337–342 - PubMed
-
- Bencharit S, Morton CL, Hyatt JL, Kuhn P, Danks MK, Potter PM, Redinbo MR. (2003) Crystal structure of human carboxylesterase 1 complexed with the Alzheimer's drug tacrine: from binding promiscuity to selective inhibition. Chem Biol 10:341–349 - PubMed
-
- Brünger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, et al. (1998) Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr 54:905–921 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

