CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts
- PMID: 20051626
- PMCID: PMC2810075
- DOI: 10.1172/JCI39397
CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts
Abstract
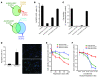
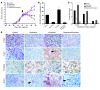
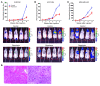
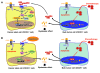
Recent evidence suggests that breast cancer and other solid tumors possess a rare population of cells capable of extensive self-renewal that contribute to metastasis and treatment resistance. We report here the development of a strategy to target these breast cancer stem cells (CSCs) through blockade of the IL-8 receptor CXCR1. CXCR1 blockade using either a CXCR1-specific blocking antibody or repertaxin, a small-molecule CXCR1 inhibitor, selectively depleted the CSC population in 2 human breast cancer cell lines in vitro. Furthermore, this was followed by the induction of massive apoptosis in the bulk tumor population via FASL/FAS signaling. The effects of CXCR1 blockade on CSC viability and on FASL production were mediated by the FAK/AKT/FOXO3A pathway. In addition, repertaxin was able to specifically target the CSC population in human breast cancer xenografts, retarding tumor growth and reducing metastasis. Our data therefore suggest that CXCR1 blockade may provide a novel means of targeting and eliminating breast CSCs.
Figures


References
-
- Phillips TM, McBride WH, Pajonk F. The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. J Natl Cancer Inst. 2006;98(24):1777–1785. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

