The therapeutic promise of the cancer stem cell concept
- PMID: 20051635
- PMCID: PMC2798700
- DOI: 10.1172/JCI41004
The therapeutic promise of the cancer stem cell concept
Abstract
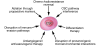
Cancer stem cells (CSCs) are a subpopulation of tumor cells that selectively possess tumor initiation and self-renewal capacity and the ability to give rise to bulk populations of nontumorigenic cancer cell progeny through differentiation. As we discuss here, they have been prospectively identified in several human malignancies, and their relative abundance in clinical cancer specimens has been correlated with malignant disease progression in human patients. Furthermore, recent findings suggest that clinical cancer progression driven by CSCs may contribute to the failure of existing therapies to consistently eradicate malignant tumors. Therefore, CSC-directed therapeutic approaches might represent translationally relevant strategies to improve clinical cancer therapy, in particular for those malignancies that are currently refractory to conventional anticancer agents directed predominantly at tumor bulk populations.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

