Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes
- PMID: 20051958
- PMCID: PMC2816662
- DOI: 10.1038/sj.bjc.6605498
Erythropoiesis-stimulating agents in oncology: a study-level meta-analysis of survival and other safety outcomes
Abstract
Background: Cancer patients often develop the potentially debilitating condition of anaemia. Numerous controlled studies indicate that erythropoiesis-stimulating agents (ESAs) can raise haemoglobin levels and reduce transfusion requirements in anaemic cancer patients receiving chemotherapy. To evaluate recent safety concerns regarding ESAs, we carried out a meta-analysis of controlled ESA oncology trials to examine whether ESA use affects survival, disease progression and risk of venous-thromboembolic events.
Methods: This meta-analysis included studies from the 2006 Cochrane meta-analysis, studies published/updated since the 2006 Cochrane report, and unpublished trial data from Amgen and Centocor Ortho Biotech. The 60 studies analysed (15 323 patients) were conducted in the settings of chemotherapy/radiochemotherapy, radiotherapy only treatment or anaemia of cancer. Data were summarised using odds ratios (ORs) with 95% confidence intervals (CIs).
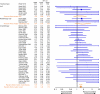
Results: Results indicated that ESA use did not significantly affect mortality (60 studies: OR=1.06; 95% CI: 0.97-1.15) or disease progression (26 studies: OR=1.01; 95% CI: 0.90-1.14), but increased the risk for venous-thromoboembolic events (44 studies: OR=1.48; 95% CI: 1.28-1.72).
Conclusion: Though this meta-analysis showed no significant effect of ESAs on survival or disease progression, prospectively designed, future randomised clinical trials will further examine the safety and efficacy of ESAs when used according to the revised labelling information.
Figures



References
-
- Aapro M, Leonard RC, Barnadas A, Marangolo M, Untch M, Malamos N, Mayordomo J, Reichert D, Pedrini JL, Ukarma L, Scherhag A, Burger HU (2008a) Effect of once-weekly epoetin beta on survival in patients with metastatic breast cancer receiving anthracycline- and/or taxane-based chemotherapy: results of the Breast Cancer-Anemia and the Value of Erythropoietin (BRAVE) study. J Clin Oncol 26: 592–598 - PubMed
-
- Abels R (1993) Erythropoietin for anaemia in cancer patients. Eur J Cancer 29A(Suppl 2): S2–S8 - PubMed
-
- Acs G, Acs P, Beckwith SM, Pitts RL, Clements E, Wong K, Verma A (2001) Erythropoietin and erythropoietin receptor expression in human cancer. Cancer Res 61: 3561–3565 - PubMed

