Detection of Mycolactone A/B in Mycobacterium ulcerans-Infected Human Tissue
- PMID: 20052267
- PMCID: PMC2791843
- DOI: 10.1371/journal.pntd.0000577
Detection of Mycolactone A/B in Mycobacterium ulcerans-Infected Human Tissue
Abstract
Background: Mycobacterium ulcerans disease (Buruli ulcer) is a neglected tropical disease common amongst children in rural West Africa. Animal experiments have shown that tissue destruction is caused by a toxin called mycolactone.
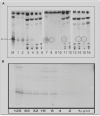
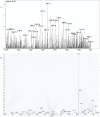
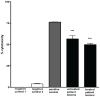
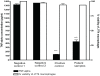
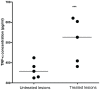
Methodology/principal findings: A molecule was identified among acetone-soluble lipid extracts from M. ulcerans (Mu)-infected human lesions with chemical and biological properties of mycolactone A/B. On thin layer chromatography this molecule had a retention factor value of 0.23, MS analyses showed it had an m/z of 765.6 [M+Na(+)] and on MS:MS fragmented to produce the core lactone ring with m/z of 429.4 and the polyketide side chain of mycolactone A/B with m/z of 359.2. Acetone-soluble lipids from lesions demonstrated significant cytotoxic, pro-apoptotic and anti-inflammatory activities on cultured fibroblast and macrophage cell lines. Mycolactone A/B was detected in all of 10 tissue samples from patients with ulcerative and pre-ulcerative Mu disease.
Conclusions/significance: Mycolactone can be detected in human tissue infected with Mu. This could have important implications for successful management of Mu infection by antibiotic treatment but further studies are needed to measure its concentration.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
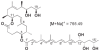

Similar articles
-
Heterogeneity of mycolactones produced by clinical isolates of Mycobacterium ulcerans: implications for virulence.Infect Immun. 2003 Feb;71(2):774-83. doi: 10.1128/IAI.71.2.774-783.2003. Infect Immun. 2003. PMID: 12540557 Free PMC article.
-
Kinetics of mycolactone in human subcutaneous tissue during antibiotic therapy for Mycobacterium ulcerans disease.BMC Infect Dis. 2014 Apr 15;14:202. doi: 10.1186/1471-2334-14-202. BMC Infect Dis. 2014. PMID: 24731247 Free PMC article.
-
Mycolactone gene expression is controlled by strong SigA-like promoters with utility in studies of Mycobacterium ulcerans and buruli ulcer.PLoS Negl Trop Dis. 2009 Nov 24;3(11):e553. doi: 10.1371/journal.pntd.0000553. PLoS Negl Trop Dis. 2009. PMID: 19936295 Free PMC article.
-
Exploring Mycolactone-The Unique Causative Toxin of Buruli Ulcer: Biosynthetic, Synthetic Pathways, Biomarker for Diagnosis, and Therapeutic Potential.Toxins (Basel). 2024 Dec 6;16(12):528. doi: 10.3390/toxins16120528. Toxins (Basel). 2024. PMID: 39728786 Free PMC article. Review.
-
Recent advances: role of mycolactone in the pathogenesis and monitoring of Mycobacterium ulcerans infection/Buruli ulcer disease.Cell Microbiol. 2016 Jan;18(1):17-29. doi: 10.1111/cmi.12547. Cell Microbiol. 2016. PMID: 26572803 Free PMC article. Review.
Cited by
-
Understanding the Significance of Biochemistry in the Storage, Handling, Purification, and Sampling of Amphiphilic Mycolactone.Toxins (Basel). 2019 Apr 4;11(4):202. doi: 10.3390/toxins11040202. Toxins (Basel). 2019. PMID: 30987300 Free PMC article.
-
Microbiological, histological, immunological, and toxin response to antibiotic treatment in the mouse model of Mycobacterium ulcerans disease.PLoS Negl Trop Dis. 2013;7(3):e2101. doi: 10.1371/journal.pntd.0002101. Epub 2013 Mar 14. PLoS Negl Trop Dis. 2013. PMID: 23516649 Free PMC article.
-
Combined inflammatory and metabolic defects reflected by reduced serum protein levels in patients with Buruli ulcer disease.PLoS Negl Trop Dis. 2014 Apr 10;8(4):e2786. doi: 10.1371/journal.pntd.0002786. eCollection 2014 Apr. PLoS Negl Trop Dis. 2014. PMID: 24722524 Free PMC article.
-
Spontaneous clearance of a secondary Buruli ulcer lesion emerging ten months after completion of chemotherapy--a case report from Togo.PLoS Negl Trop Dis. 2012;6(7):e1747. doi: 10.1371/journal.pntd.0001747. Epub 2012 Jul 31. PLoS Negl Trop Dis. 2012. PMID: 22860147 Free PMC article. No abstract available.
-
Detection of Mycolactone by Thin Layer Chromatography.Methods Mol Biol. 2022;2387:131-149. doi: 10.1007/978-1-0716-1779-3_14. Methods Mol Biol. 2022. PMID: 34643909
References
-
- Wansbrough-Jones M, Phillips R. Buruli ulcer: emerging from obscurity. Lancet. 2006;367:1849–1858. - PubMed
-
- Evans MR, Phillips R, Etuaful SN, Amofah G, Adomako J, et al. An outreach education and treatment project in Ghana for the early stage of Mycobacterium ulcerans disease. Trans R Soc Trop Med Hyg. 2003;97:159–160. - PubMed
-
- Connor DH, Lunn HF. Mycobacterium ulcerans infection (with comments on pathogenesis). Int J Lepr. 1965;33(Suppl):698–709. - PubMed
-
- Pimsler M, Sponsler TA, Meyers WM. Immunosuppressive properties of the soluble toxin from Mycobacterium ulcerans. J Infect Dis. 1988;157:577–580. - PubMed
-
- Gunawardana G, Chatterjee D, George KM, Brennan P, Whittern D, et al. Mycolactone A and B: toxins of Mycobacterium ulcerans. J Am Chem Soc. 1999;121:6092–6093.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

