Interferon-inducible IFI16, a negative regulator of cell growth, down-regulates expression of human telomerase reverse transcriptase (hTERT) gene
- PMID: 20052289
- PMCID: PMC2797294
- DOI: 10.1371/journal.pone.0008569
Interferon-inducible IFI16, a negative regulator of cell growth, down-regulates expression of human telomerase reverse transcriptase (hTERT) gene
Abstract
Background: Increased levels of interferon (IFN)-inducible IFI16 protein (encoded by the IFI16 gene located at 1q22) in human normal prostate epithelial cells and diploid fibroblasts (HDFs) are associated with the onset of cellular senescence. However, the molecular mechanisms by which the IFI16 protein contributes to cellular senescence-associated cell growth arrest remain to be elucidated. Here, we report that increased levels of IFI16 protein in normal HDFs and in HeLa cells negatively regulate the expression of human telomerase reverse transcriptase (hTERT) gene.
Methodology/principal findings: We optimized conditions for real-time PCR, immunoblotting, and telomere repeat amplification protocol (TRAP) assays to detect relatively low levels of hTERT mRNA, protein, and telomerase activity that are found in HDFs. Using the optimized conditions, we report that treatment of HDFs with inhibitors of cell cycle progression, such as aphidicolin or CGK1026, which resulted in reduced steady-state levels of IFI16 mRNA and protein, was associated with increases in hTERT mRNA and protein levels and telomerase activity. In contrast, knockdown of IFI16 expression in cells increased the expression of c-Myc, a positive regulator of hTERT expression. Additionally, over-expression of IFI16 protein in cells inhibited the c-Myc-mediated stimulation of the activity of hTERT-luc-reporter and reduced the steady-state levels of c-Myc and hTERT.
Conclusions/significance: These data demonstrated that increased levels of IFI16 protein in HDFs down-regulate the expression of hTERT gene. Our observations will serve basis to understand how increased cellular levels of the IFI16 protein may contribute to certain aging-dependent diseases.
Conflict of interest statement
Figures




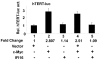

Similar articles
-
Activation of telomerase by human cytomegalovirus.J Natl Cancer Inst. 2009 Apr 1;101(7):488-97. doi: 10.1093/jnci/djp031. Epub 2009 Mar 24. J Natl Cancer Inst. 2009. PMID: 19318640
-
Regulation of human telomerase activity: repression by normal chromosome 3 abolishes nuclear telomerase reverse transcriptase transcripts but does not affect c-Myc activity.Cancer Res. 2001 Oct 15;61(20):7594-602. Cancer Res. 2001. PMID: 11606399
-
Differential roles for the interferon-inducible IFI16 and AIM2 innate immune sensors for cytosolic DNA in cellular senescence of human fibroblasts.Mol Cancer Res. 2011 May;9(5):589-602. doi: 10.1158/1541-7786.MCR-10-0565. Epub 2011 Apr 6. Mol Cancer Res. 2011. PMID: 21471287 Free PMC article.
-
Interferon-inducible IFI16 protein in human cancers and autoimmune diseases.Front Biosci. 2008 Jan 1;13:598-608. doi: 10.2741/2705. Front Biosci. 2008. PMID: 17981573 Review.
-
Transcriptional regulation of the telomerase hTERT gene as a target for cellular and viral oncogenic mechanisms.Carcinogenesis. 2003 Jul;24(7):1167-76. doi: 10.1093/carcin/bgg085. Epub 2003 May 22. Carcinogenesis. 2003. PMID: 12807729 Review.
Cited by
-
Regulation of cellular senescence by innate immunity.Biophys Rep. 2023 Dec 31;9(6):338-351. doi: 10.52601/bpr.2023.230032. Biophys Rep. 2023. PMID: 38524701 Free PMC article.
-
Type I interferons and related pathways in cell senescence.Aging Cell. 2020 Oct;19(10):e13234. doi: 10.1111/acel.13234. Epub 2020 Sep 12. Aging Cell. 2020. PMID: 32918364 Free PMC article. Review.
-
IFI16 Expression Is Related to Selected Transcription Factors during B-Cell Differentiation.J Immunol Res. 2015;2015:747645. doi: 10.1155/2015/747645. Epub 2015 Jun 22. J Immunol Res. 2015. PMID: 26185770 Free PMC article.
-
The functional interactome of PYHIN immune regulators reveals IFIX is a sensor of viral DNA.Mol Syst Biol. 2015 Feb 9;11(1):787. doi: 10.15252/msb.20145808. Mol Syst Biol. 2015. PMID: 25665578 Free PMC article.
-
Role of the HIN domain in regulation of innate immune responses.Mol Cell Biol. 2014 Jan;34(1):2-15. doi: 10.1128/MCB.00857-13. Epub 2013 Oct 28. Mol Cell Biol. 2014. PMID: 24164899 Free PMC article. Review.
References
-
- Borden EC, Lindner D, Dreicer R, Hussein M, Peereboom D. Second-generation interferons for cancer: clinical targets. Semin Cancer Biol. 2000;10:125–144. - PubMed
-
- van Boxel-Dezaire AH, Rani MR, Stark GR. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity. 2006;25:361–372. - PubMed
-
- Garcia-Sastre A, Biron CA. Type I interferons and the virus-host relationship: a lesson in détente. Science. 2006;312:879–882. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

