Mild hypoxia enhances proliferation and multipotency of human neural stem cells
- PMID: 20052410
- PMCID: PMC2797394
- DOI: 10.1371/journal.pone.0008575
Mild hypoxia enhances proliferation and multipotency of human neural stem cells
Abstract
Background: Neural stem cells (NSCs) represent an optimal tool for studies and therapy of neurodegenerative diseases. We recently established a v-myc immortalized human NSC (IhNSC) line, which retains stem properties comparable to parental cells. Oxygen concentration is one of the most crucial environmental conditions for cell proliferation and differentiation both in vitro and in vivo. In the central nervous system, physiological concentrations of oxygen range from 0.55 to 8% oxygen. In particular, in the in the subventricular zone niche area, it's estimated to be 2.5 to 3%.
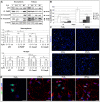
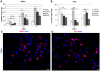
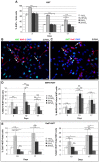
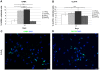
Methodology/principal findings: We investigated in vitro the effects of 1, 2.5, 5, and 20% oxygen concentrations on IhNSCs both during proliferation and differentiation. The highest proliferation rate, evaluated through neurosphere formation assay, was obtained at 2.5 and 5% oxygen, while 1% oxygen was most noxious for cell survival. The differentiation assays showed that the percentages of beta-tubIII+ or MAP2+ neuronal cells and of GalC+ oligodendrocytes were significantly higher at 2.5% compared with 1, 5, or 20% oxygen at 17 days in vitro. Mild hypoxia (2.5 to 5% oxygen) promoted differentiation into neuro-oligodendroglial progenitors as revealed by the higher percentage of MAP2+/Ki67+ and GalC+/Ki67+ residual proliferating progenitors, and enhanced the yield of GABAergic and slightly of glutamatergic neurons compared to 1% and 20% oxygen where a significant percentage of GFAP+/nestin+ cells were still present at 17 days of differentiation.
Conclusions/significance: These findings raise the possibility that reduced oxygen levels occurring in neuronal disorders like cerebral ischemia transiently lead to NSC remaining in a state of quiescence. Conversely, mild hypoxia favors NSC proliferation and neuronal and oligodendroglial differentiation, thus providing an important advance and a useful tool for NSC-mediated therapy of ischemic stroke and neurodegenerative diseases like Parkinson's disease, multiple sclerosis, and Alzheimer's disease.
Conflict of interest statement
Figures


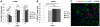

References
-
- Vescovi AL, Parati EA, Gritti A, Poulin P, Ferrario M, et al. Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Exp Neurol. 1999;156:71–83. - PubMed
-
- McKay R. Stem cells in the central nervous system. Science. 1997;276:66–71. - PubMed
-
- Gritti A, Cova L, Parati EA, Galli R, Vescovi AL. Basic fibroblast growth factor supports the proliferation of epidermal growth factor-generated neuronal precursor cells of the adult mouse CNS. Neurosci Lett. 1995;185:151–154. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

