Myosin II motor proteins with different functions determine the fate of lamellipodia extension during cell spreading
- PMID: 20052411
- PMCID: PMC2797395
- DOI: 10.1371/journal.pone.0008560
Myosin II motor proteins with different functions determine the fate of lamellipodia extension during cell spreading
Abstract
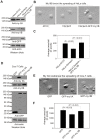
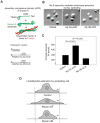
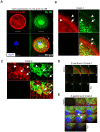
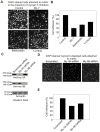
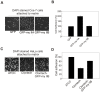
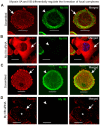
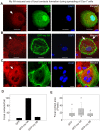
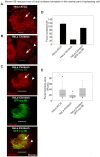
Non-muscle cells express multiple myosin-II motor proteins myosin IIA, myosin IIB and myosin IIC transcribed from different loci in the human genome. Due to a significant homology in their sequences, these ubiquitously expressed myosin II motor proteins are believed to have overlapping cellular functions, but the mechanistic details are not elucidated. The present study uncovered a mechanism that coordinates the distinctly localized myosin IIA and myosin IIB with unexpected opposite mechanical roles in maneuvering lamellipodia extension, a critical step in the initiation of cell invasion, spreading, and migration. Myosin IIB motor protein by localizing at the front drives lamellipodia extension during cell spreading. On the other hand, myosin IIA localizes next to myosin IIB and attenuates or retracts lamellipodia extension. Myosin IIA and IIB increase cell adhesion by regulating focal contacts formation in the spreading margins and central part of the spreading cell, respectively. Spreading cells expressing both myosin IIA and myosin IIB motor proteins display an organized actin network consisting of retrograde filaments, arcs and central filaments attached to focal contacts. This organized actin network especially arcs and focal contacts formation in the spreading margins were lost in myosin IIA cells. Surprisingly, myosin IIB cells displayed long parallel actin filaments connected to focal contacts in the spreading margins. Thus, with different roles in the regulation of the actin network and focal contacts formation, both myosin IIA and IIB determine the fate of lamellipodia extension during cell spreading.
Conflict of interest statement
Figures

References
-
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. - PubMed
-
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;12:453–465. - PubMed
-
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. - PubMed
-
- Giannone G, Dubin-Thaler BJ, Dobereiner HG, Kieffer N, Bresnick AR, et al. Periodic lamellipodial contractions correlate with rearward actin waves. Cell. 2004;116:431–443. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

