A trial of 3 interventions to promote colorectal cancer screening in African Americans
- PMID: 20052732
- PMCID: PMC2819540
- DOI: 10.1002/cncr.24842
A trial of 3 interventions to promote colorectal cancer screening in African Americans
Abstract
Background: Colorectal cancer (CRC) is the second leading cause of cancer death in the United States. CRC incidence and mortality rates are higher among blacks than among whites, and screening rates are lower in blacks than in whites. For the current study, the authors tested 3 interventions that were intended to increase the rate of CRC screening among African Americans.
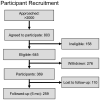
Methods: The following interventions were chosen to address evidence gaps in the Centers for Disease Control and Prevention's Guide to Community Preventive Services: one-on-one education, group education, and reducing out-of-pocket costs. Three hundred sixty-nine African-American men and women aged > or =50 years were enrolled in this randomized, controlled community intervention trial. The main outcome measures were postintervention increase in CRC knowledge and obtaining a screening test within 6 months.
Results: There was substantial attrition: Two hundred fifty-seven participants completed the intervention and were available for follow-up 3 months to 6 months later. Among completers, there were significant increases in knowledge in both educational cohorts but in neither of the other 2 cohorts. By the 6-month follow-up, 17.7% (11 of 62 participants) of the Control cohort reported having undergone screening compared with 33.9% (22 of 65 participants) of the Group Education cohort (P = .039). Screening rate increases in the other 2 cohorts were not statistically significant.
Conclusions: The current results indicated that group education could increase CRC cancer screening rates among African Americans. The screening rate of <35% in a group of individuals who participated in an educational program through multiple sessions over a period of several weeks indicated that there still are barriers to overcome.
References
-
- MMWR Morb Mortal Wkly Rep. 2006. pp. 308–311. www.cdc.gov/mmwr/preview/mmwrhtml/mm551a4.htm. - PubMed
-
- American Cancer Society. 2007. www.CAFF2007AAacspdf2007.pdf.
-
- National Cancer Institute, Division of Cancer Control and Population Sciences. Surveillance Research. http://surveillance.cancer.gov/statistics/types/mortality.html.
-
- Pignone M, Rich M, Teutsch SM, Berg AO, Lohr KN. Screening for colorectal cancer in adults at average risk: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;137:132–41. - PubMed
Publication types
MeSH terms
Grants and funding
- U54 MD007588/MD/NIMHD NIH HHS/United States
- U54 CA118638/CA/NCI NIH HHS/United States
- 2U54CA118638/CA/NCI NIH HHS/United States
- R01 CA166785/CA/NCI NIH HHS/United States
- G12 MD007585/MD/NIMHD NIH HHS/United States
- UL1RR025008/RR/NCRR NIH HHS/United States
- U01 CA114652/CA/NCI NIH HHS/United States
- UL1 RR025008/RR/NCRR NIH HHS/United States
- 1UL1RR025008/RR/NCRR NIH HHS/United States
- 5U48DP000049/DP/NCCDPHP CDC HHS/United States
- U57CCU42068/PHS HHS/United States
- U54 CA118623/CA/NCI NIH HHS/United States
- U48 DP000049/DP/NCCDPHP CDC HHS/United States
- U54 CA118948/CA/NCI NIH HHS/United States
- UL1 TR000454/TR/NCATS NIH HHS/United States
- 1U01CA1146520/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical