Characterization of a covalent polysulfane bridge in copper-zinc superoxide dismutase
- PMID: 20052996
- PMCID: PMC2819567
- DOI: 10.1021/bi901844d
Characterization of a covalent polysulfane bridge in copper-zinc superoxide dismutase
Abstract
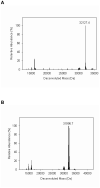
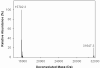
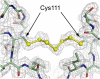
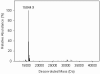
In the course of studies on human copper-zinc superoxide dismutase (SOD1), we observed a modified form of the protein whose mass was increased by 158 mass units. The covalent modification was characterized, and we established that it is a novel heptasulfane bridge connecting the two Cys111 residues in the SOD1 homodimer. The heptasulfane bridge was visualized directly in the crystal structure of a recombinant human mutant SOD1, H46R/H48Q, produced in yeast. The modification is reversible, with the bridge being cleaved by thiols, by cyanide, and by unfolding of the protein to expose the polysulfane. The polysulfane bridge can be introduced in vitro by incubation of purified SOD1 with elemental sulfur, even under anaerobic conditions and in the presence of a metal chelator. Because polysulfanes and polysulfides can catalyze the generation of reactive oxygen and sulfur species, the modification may endow SOD1 with a toxic gain of function.
Figures



Similar articles
-
Dynamical roles of metal ions and the disulfide bond in Cu, Zn superoxide dismutase folding and aggregation.Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19696-701. doi: 10.1073/pnas.0803266105. Epub 2008 Dec 3. Proc Natl Acad Sci U S A. 2008. PMID: 19052230 Free PMC article.
-
Structural and biophysical properties of the pathogenic SOD1 variant H46R/H48Q.Biochemistry. 2009 Apr 21;48(15):3436-47. doi: 10.1021/bi8021735. Biochemistry. 2009. PMID: 19227972 Free PMC article.
-
Variable metallation of human superoxide dismutase: atomic resolution crystal structures of Cu-Zn, Zn-Zn and as-isolated wild-type enzymes.J Mol Biol. 2006 Mar 10;356(5):1152-62. doi: 10.1016/j.jmb.2005.11.081. Epub 2005 Dec 12. J Mol Biol. 2006. PMID: 16406071
-
Oxidative modification to cysteine sulfonic acid of Cys111 in human copper-zinc superoxide dismutase.J Biol Chem. 2007 Dec 7;282(49):35933-44. doi: 10.1074/jbc.M702941200. Epub 2007 Oct 3. J Biol Chem. 2007. PMID: 17913710
-
Copper-zinc superoxide dismutase and amyotrophic lateral sclerosis.Annu Rev Biochem. 2005;74:563-93. doi: 10.1146/annurev.biochem.72.121801.161647. Annu Rev Biochem. 2005. PMID: 15952898 Review.
Cited by
-
Cysteine Metabolism in Neuronal Redox Homeostasis.Trends Pharmacol Sci. 2018 May;39(5):513-524. doi: 10.1016/j.tips.2018.02.007. Epub 2018 Mar 9. Trends Pharmacol Sci. 2018. PMID: 29530337 Free PMC article. Review.
-
Serum total oxidant/antioxidant status and trace element levels in breast cancer patients.Int J Clin Oncol. 2012 Dec;17(6):575-83. doi: 10.1007/s10147-011-0327-y. Epub 2011 Oct 5. Int J Clin Oncol. 2012. PMID: 21968912
-
Altered thiol chemistry in human amyotrophic lateral sclerosis-linked mutants of superoxide dismutase 1.J Biol Chem. 2014 Sep 26;289(39):26722-26732. doi: 10.1074/jbc.M114.565333. Epub 2014 Aug 4. J Biol Chem. 2014. PMID: 25096579 Free PMC article.
-
3-Mercaptopyruvate sulfurtransferase produces potential redox regulators cysteine- and glutathione-persulfide (Cys-SSH and GSSH) together with signaling molecules H2S2, H2S3 and H2S.Sci Rep. 2017 Sep 5;7(1):10459. doi: 10.1038/s41598-017-11004-7. Sci Rep. 2017. PMID: 28874874 Free PMC article.
-
AH-DB: collecting protein structure pairs before and after binding.Nucleic Acids Res. 2012 Jan;40(Database issue):D472-8. doi: 10.1093/nar/gkr940. Epub 2011 Nov 13. Nucleic Acids Res. 2012. PMID: 22084200 Free PMC article.
References
-
- Fridovich I. Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 1995;64:97–112. 97-112. - PubMed
-
- Tiwari A, Hayward LJ. Familial amyotrophic lateral sclerosis mutants of copper/zinc superoxide dismutase are susceptible to disulfide reduction. J Biol. Chem. 2003;278:5984–5992. - PubMed
-
- Arnesano F, Banci L, Bertini I, Martinelli M, Furukawa Y, O'Halloran TV. The unusually stable quaternary structure of human Cu,Zn-superoxide dismutase 1 is controlled by both metal occupancy and disulfide status. J. Biol. Chem. 2004;279:47998–48003. - PubMed
-
- Culotta VC, Klomp LW, Strain J, Casareno RL, Krems B, Gitlin JD. The copper chaperone for superoxide dismutase. J. Biol. Chem. 1997;272:23469–23472. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

