Growth and differentiation of the larval mosquito midgut
- PMID: 20053117
- PMCID: PMC3011905
- DOI: 10.1673/031.009.5501
Growth and differentiation of the larval mosquito midgut
Abstract
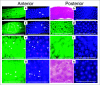
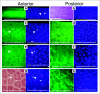
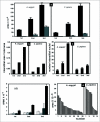
Factors affecting larval growth and nutrition have consequences on adult fecundity. Since the mosquito larval midgut is the primary organ of digestion and nutrient absorption, factors that affect the growth and development of the midgut may have potential consequences on the reproductive potential of the adult. To gain a better understanding of mosquito midgut development the growth and metamorphic remodeling of the Aedes aegypti L. and Culex pipiens L. (Diptera: Culicidae) midguts were investigated. Cytological evidence was obtained suggesting that, in both the anterior and posterior Ae. aegypti larval midgut, diploid regenerative cells give rise to new endoreplicating cells that significantly contribute to the growth and metabolism of the midgut. This hypothesis was supported by BrdU incorporation studies showing that diploid cells, as well as large and small endoreplicating cells, synthesize DNA during the 2(nd), 3(rd) and 4(th) instars. Cytological studies of the Cx. pipiens larval midgut suggest that anterior midgut growth in this species is primarily by cell enlargement. To study metamorphic remodeling of the midgut, DNA synthesis in Ae. aegypti 4(th) instar midguts was followed by using 5-bromo-2-deoxyuridine (BrdU) incorporation. During the 24 hr period after the last larval-larval molt both endoreplicating and diploid cells incorporate BrdU. After the critical weight is achieved, endoreplicating cell BrdU incorporation gradually ceases while diploid cells continue to replicate. The period of maximum diploid cell incorporation correlated with the period of maximum ecdysone titer.
Figures


References
-
- Baldwin KM, Hakim RS. Growth and differentiation of the larval midgut epithelium during molting in the moth, Manduca sexta. . Tissue and Cell. 1991;23:411–422. - PubMed
-
- Berger CA. Cytology of metamorphosis in the Culininae. Nature. 1938;141:834–835.
-
- Briegel H. Fecundity, metabolism, and body size in Anopheles (Diptera: Culicidae), vectors of malaria. Journal of Medical Entomology. 1990;27:839–850. - PubMed
-
- Briegel H. Physiological basis of mosquito ecology. Journal of Vector Ecology. 2003;28:1–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

