Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance
- PMID: 20053163
- PMCID: PMC2908937
- DOI: 10.2174/138620710790596736
Building a tiered approach to in vitro predictive toxicity screening: a focus on assays with in vivo relevance
Abstract
One of the greatest challenges facing the pharmaceutical industry today is the failure of promising new drug candidates due to unanticipated adverse effects discovered during preclinical animal safety studies and clinical trials. Late stage attrition increases the time required to bring a new drug to market, inflates development costs, and represents a major source of inefficiency in the drug discovery/development process. It is generally recognized that early evaluation of new drug candidates is necessary to improve the process. Building in vitro data sets that can accurately predict adverse effects in vivo would allow compounds with high risk profiles to be deprioritized, while those that possess the requisite drug attributes and a lower risk profile are brought forward. In vitro cytotoxicity assays have been used for decades as a tool to understand hypotheses driven questions regarding mechanisms of toxicity. However, when used in a prospective manner, they have not been highly predictive of in vivo toxicity. Therefore, the issue may not be how to collect in vitro toxicity data, but rather how to translate in vitro toxicity data into meaningful in vivo effects. This review will focus on the development of an in vitro toxicity screening strategy that is based on a tiered approach to data collection combined with data interpretation.
Figures




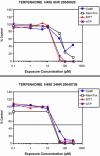
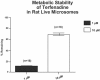




References
-
- Kane MD. 3rd International Symposium on Early Toxicity Screening. Expert Opin. Drug Saf. 2003;2:199–201. - PubMed
-
- Kola I, Landis J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. 2004;3:711–715. - PubMed
-
- Wienkers LC, Heath TG. Predicting in vivo drug interactions from in vitro drug discovery data. Nat. Rev. Drug Dis. 2005;4:825–833. - PubMed
-
- Ito K, Hallifax D, Obach RS, Houston JB. Impact of parallel pathways of drug elimination and multiple cytochrome P450 involvement on drug-drug interactions: CYP2D6 paradigm. Drug Metab. Dispos. 2005;33:837–844. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
