Variable viral clearance despite adequate ganciclovir plasma levels during valganciclovir treatment for cytomegalovirus disease in D+/R- transplant recipients
- PMID: 20053269
- PMCID: PMC2820479
- DOI: 10.1186/1471-2334-10-2
Variable viral clearance despite adequate ganciclovir plasma levels during valganciclovir treatment for cytomegalovirus disease in D+/R- transplant recipients
Abstract
Background: Valganciclovir, the oral prodrug of ganciclovir, has been demonstrated equivalent to iv ganciclovir for CMV disease treatment in solid organ transplant recipients. Variability in ganciclovir exposure achieved with valganciclovir could be implicated as a contributing factor for explaining variations in the therapeutic response. This prospective observational study aimed to correlate clinical and cytomegalovirus (CMV) viral load response (DNAemia) with ganciclovir plasma concentrations in patients treated with valganciclovir for CMV infection/disease.
Methods: Seven CMV D+/R- transplant recipients (4 kidney, 2 liver and 1 heart) were treated with valganciclovir (initial dose was 900-1800 mg/day for 3-6.5 weeks, followed by 450-900 mg/day for 2-9 weeks). DNAemia was monitored by real time quantitative PCR and ganciclovir plasma concentration was measured at trough (Ctrough) and 3 h after drug administration (C3h) by HPLC.
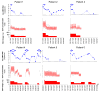
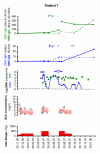
Results: Four patients presented with CMV syndrome, two had CMV tissue-invasive disease after prophylaxis discontinuation, and one liver recipient was treated pre-emptively for asymptomatic rising CMV viral load 5 weeks post-transplantation in the absence of prophylaxis. CMV DNAemia decreased during the first week of treatment in all recipients except in one patient (median decrease: -1.2 log copies/mL, range: -1.8 to 0) despite satisfactory ganciclovir exposure (AUC0-12 = 48 mgxh/L, range for the 7 patients: 40-118 mgxh/L). Viral clearance was obtained in five patients after a median of time of 34 days (range: 28-82 days). Two patients had recurrent CMV disease despite adequate ganciclovir exposure (65 mgxh/L, range: 44-118 mgxh/L).
Conclusions: Valganciclovir treatment for CMV infection/disease in D+/R- transplant recipients can thus result in variable viral clearance despite adequate ganciclovir plasma concentrations, probably correlating inversely with anti-CMV immune responses after primary infection.
Figures
References
-
- Ruttmann E, Geltner C, Bucher B, Ulmer H, Höfer D, Hangler HB, Semsroth S, Margreiter R, Laufer G, Müller LC. Combined CMV prophylaxis improves outcome and reduces the risk for bronchiolitis obliterans syndrome (BOS) after lung transplantation. Transplantation. 2006;81:1415–1420. doi: 10.1097/01.tp.0000209439.27719.ed. - DOI - PubMed
-
- Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, Freeman R, Heaton N, Pescovitz MD. Valganciclovir Solid Organ Transplant Study Group. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004;4:611–620. doi: 10.1111/j.1600-6143.2004.00382.x. - DOI - PubMed
-
- Åsberg A, Humar A, Rollag H, Jardine AG, Mouas H, Pescovitz MD, Sgarabotto D, Tuncer M, Noronha IL, Hartmann A. VICTOR Study Group. Oral valganciclovir is noninferior to intravenous ganciclovir for the treatment of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2007;7:2106–2113. doi: 10.1111/j.1600-6143.2007.01910.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

