A Rac1 inhibitory peptide suppresses antibody production and paw swelling in the murine collagen-induced arthritis model of rheumatoid arthritis
- PMID: 20053277
- PMCID: PMC2875627
- DOI: 10.1186/ar2900
A Rac1 inhibitory peptide suppresses antibody production and paw swelling in the murine collagen-induced arthritis model of rheumatoid arthritis
Abstract
Introduction: The Rho family GTPase Rac1 regulates cytoskeletal rearrangements crucial for the recruitment, extravasation and activation of leukocytes at sites of inflammation. Rac1 signaling also promotes the activation and survival of lymphocytes and osteoclasts. Therefore, we assessed the ability of a cell-permeable Rac1 carboxy-terminal inhibitory peptide to modulate disease in mice with collagen-induced arthritis (CIA).
Methods: CIA was induced in DBA/1 mice, and in either early or chronic disease, mice were treated three times per week by intraperitoneal injection with control peptide or Rac1 inhibitory peptide. Effects on disease progression were assessed by measurement of paw swelling. Inflammation and joint destruction were examined by histology and radiology. Serum levels of anti-collagen type II antibodies were measured by enzyme-linked immunosorbent assay. T-cell phenotypes and activation were assessed by fluorescence-activated cell sorting analysis. Results were analyzed using Mann-Whitney U and unpaired Student t tests.
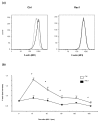
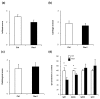
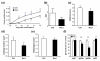
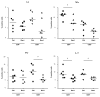
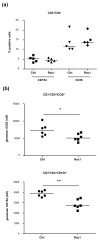
Results: Treatment of mice with Rac1 inhibitory peptide resulted in a decrease in paw swelling in early disease and to a lesser extent in more chronic arthritis. Of interest, while joint destruction was unaffected by Rac1 inhibitory peptide, anti-collagen type II antibody production was significantly diminished in treated mice, in both early and chronic arthritis. Ex vivo, Rac1 inhibitory peptide suppressed T-cell receptor/CD28-dependent production of tumor necrosis factor alpha, interferon gamma and interleukin-17 by T cells from collagen-primed mice, and reduced induction of ICOS and CD154, T-cell costimulatory proteins important for B-cell help.
Conclusions: The data suggest that targeting of Rac1 with the Rac1 carboxy-terminal inhibitory peptide may suppress T-cell activation and autoantibody production in autoimmune disease. Whether this could translate into clinically meaningful improvement remains to be shown.
Figures


Comment in
-
'Rac'-ing upstream to treat rheumatoid arthritis.Arthritis Res Ther. 2010;12(1):109. doi: 10.1186/ar2924. Epub 2010 Feb 24. Arthritis Res Ther. 2010. PMID: 20236447 Free PMC article.
References
-
- Moll J, Sansig G, Fattori E, Putten H van der. The murine rac1 gene: cDNA cloning, tissue distribution and regulated expression of rac1 mRNA by disassembly of actin microfilaments. Oncogene. 1991;6:863–866. - PubMed
-
- Shirsat NV, Pignolo RJ, Kreider BL, Rovera G. A member of the ras gene superfamily is expressed specifically in T, B and myeloid hemopoietic cells. Oncogene. 1990;5:769–772. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

